Combustion of Individual Iron Microparticles With Resolved Boundary Layers I
Introduction
Metal fuels are promising green energy carriers due to their high volumetric energy density, recyclability, and availability. Iron, in particular, has recently come into focus for researchers since it has a low market price and iron-air suspensions exhibit comparable burning velocities as conventional hydrocarbon fuels.
Previous studies have shown that the flame characteristics of iron flames are highly dependent on the ignition of the individual iron particles. In this work, the focus is on the kinetically-controlled oxidation phase prior to particle ignition, where the kinetic reaction rate is governed by the reaction kinetics of the model. Single particle simulations with fully resolved boundary layers are conducted to evaluate different models for the heterogeneous combustion of iron microparticles. Heat and mass transfer is accurately captured by resolving the particle boundary layer, such that both, kinetically-controlled and diffusion-limited combustion can be accurately recovered.
Methods
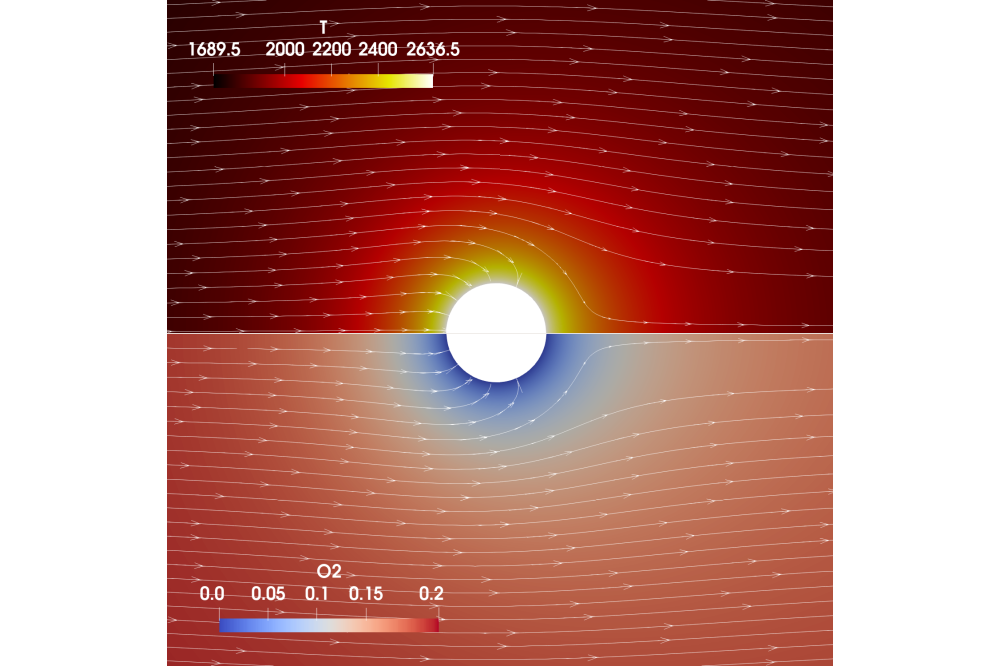
A compressible and reactive solver within the open-source code OpenFOAM-v2012 is chosen for this work, since the temperature and density gradients change tremendously in the boundary layer of a burning particle. The ability to handle chemical reactions in the boundary layer is necessary, since gaseous iron can be released which further react with theoxygen in the gas. Since iron particles are micro-sized, it is assumed that heat and mass transfer inside a particle is infinitely fast and therefore, the solid particle phase is not resolved. Instead, the particle is represented by a combustion model at the particle-to-gas interface.
Results
The computational method is able to accurately capture heat and mass transfer between the particle and the surrounding gas phase. It was validated by comparing the non-reactive numerical heat and mass transfer under different temperatures and Reynolds numbers with analytical solutions. The method is then further validated using experimental data of single particle oxidation. Under different ambient gas compositions and with different particle sizes, the combustion time of the particles is measured. Previous studies have shown that the majority of the combustion time is diffusion-limited and therefore governed by heat and mass transfer. Therefore, this data can be used to validate the accuracy of heat and mass transfer in the numerical method. In our work, excellent agreement is found.
The experimental data is then used to assess the kinetically-controlled oxidation prior to the ignition of the particles. This phase is mostly governed by the chosen combustion model. It is found that the state-of-the-art combustion models significantly underpredict the measured ignition delay time. Furthermore, the ignition is dependent on the oxygen concentration in the gas phase. This is an indication that the ignition of an iron microparticle might be limited by adsorption of oxygen molecules on the particle surface. The experimental data is then used to test modeling assumptions and calibrate the model kinetics parameters.
Discussion
With the new parameter set, the numerically obtained ignition delay time shows a better agreement with the experimental data. In future works, this could contribute to an improvement of the reaction front speed in simulations of iron suspensions. Despite the better agreement with the experimental results with the calibrated model, there are conceivably further thermophysical processes which influence the ignition of iron microparticles that are not yet known which warrant further research.