Biogenic Stabilizers: Structure – Property Relationships
Introduction
Additives are crucial for the functioning and application of plastics. They enable applications in which special properties, such as low flammability, resistance to certain influences, or targeted decomposition, are required. The most important additives are antioxidants, which protect polymers from oxygen and thermally induced degradation, often caused by the formation of peroxides and hydroperoxides. These peroxides lead to the degradation of polymer chains and a decrease in plastic properties, such as mechanical stability. During decomposition, new peroxides are formed, resulting in a self-accelerating autoxidation cycle.
One class of antioxidants are sterically hindered phenolic structures. They act as H-donors and react preferentially with peroxides to deactivate them. The phenols themselves carry the free electron, which can be stabilized by delocalization through the aromatic system.
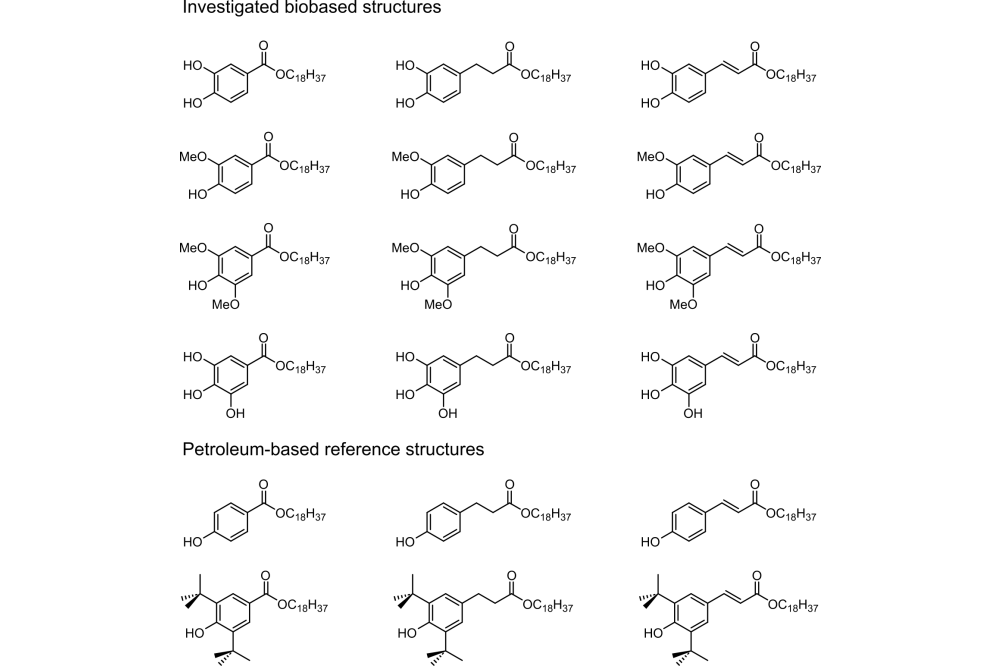
One way to investigate antioxidant behavior is the DPPH assay, in which the stable DPPH radical reacts with the potential antioxidant to demonstrate radical quenching ability. Since the DPPH radical and the DPPH-H species have different absorption spectra, efficiency can be extracted from UV-Vis experiments. Another test to determine the processing stabilization properties of antioxidants can be performed in a micro compounder. In this process, stabilizers are mixed with virgin polypropylene and fed into the micro compounder for 30 minutes to simulate multiple extrusion steps. During this time, the screw torque of the compounder, which is proportional to polymer viscosity and chain length, is monitored. The more the screw torque decreases, the less efficient the antioxidant. We investigated several hydroxy-, methoxy- , tert-butyl, and non-substituted benzoates, cinnamates, and phenyl propionates, as shown in Figure 1, to gain deeper insights into structure-property relationships. To achieve this, we used High Performance Computers to calculate and predict the antioxidant properties of different benzoates, cinnamates, and phenyl propionates
Methods
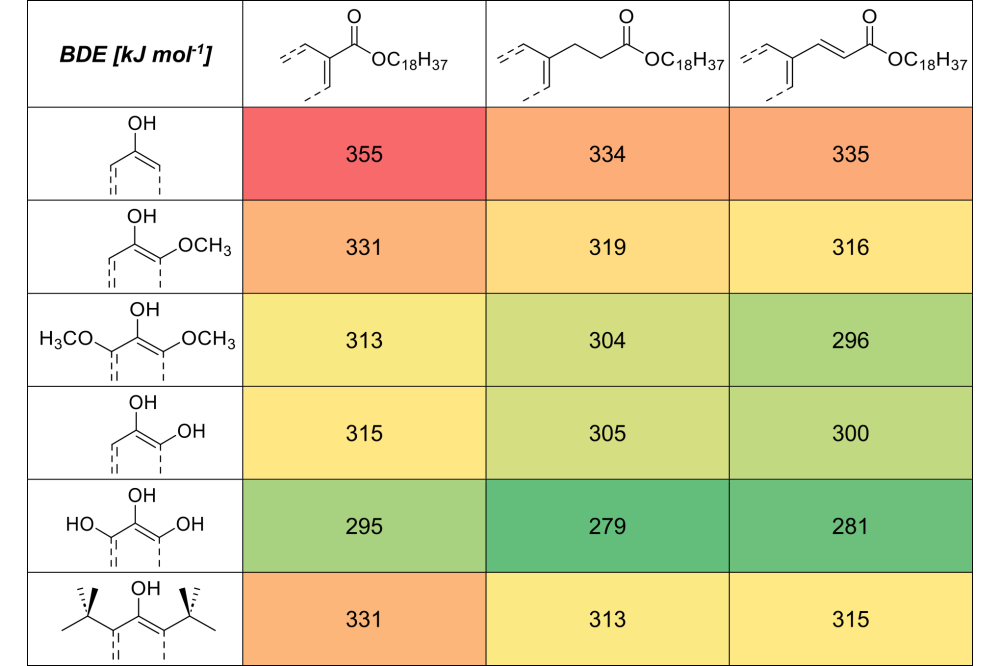
We employed closed-shell and unrestricted open-shell DFT optimizations, both with and without solvent simulations (CPCM method), on the anions, cations, neutral, and radical forms of the investigated stabilizers to obtain individual energies. Using these energies, we calculated the bond dissociation energy (BDE), ionization potential (IP), proton dissociation enthalpy (PDE), proton affinity (PA), and electron transfer energy (ETE) of the investigated structures.
Results
Antioxidants in DPPH assays can react via different pathways, including Hydrogen Atom Transfer (HAT), Proton Transfer (PT), Single Electron Transfer (SET), Sequential Proton Loss Electron Transfer (SPLET), and Single Electron Transfer-Proton Transfer (SET-PT). These mechanisms depend on factors such as bond dissociation energy (BDE), ionization potential (IP), and solvent properties. Multiple mechanisms can occur simultaneously, with one typically dominating based on the conditions.
Another point is the steric hindrance and substitution in the para-position of the phenols, as these significantly affect radical scavenging activity. Here, we investigated hydroxy-, methoxy- , tert-butyl, and non-substituted structures to find structure-property relationships.
Discussion
The results of the DPPH assays closely align with those from the micro compounder experiments, indicating that while certain conditions apply, the DPPH assay serves as a reliable preliminary assessment of antioxidant efficacy during processing. In general, hydroxy-substituted structures perform better than methoxy-substituted ones, while reference structures without additional groups show poor radical efficiency. This supports the notion that steric hindrance and electronic effects play crucial roles in antioxidant behavior. Structures with multiple hydroxyl groups exhibit significantly lower bond dissociation energies (BDE), facilitating faster hydrogen transfer and enhanced stabilization performance during polypropylene processing.
The analysis of various substance classes – benzoates, cinnamates, and phenyl propionates – reveals distinct differences in their stabilizing effects. Benzoates, with their direct ester groups, exhibit lower radical efficiency due to higher bond dissociation energies (BDE). In contrast, cinnamates benefit from extended conjugation, which enhances radical stabilization and results in improved performance. Phenyl propionates, characterized by a flexible alkyl chain, not only lower BDE but also facilitate easier hydrogen transfer during stabilization, making them the most effective class in this study. They stand out due to their ability to undergo disproportionation, a process that allows them to regenerate partially and form additional stabilizing phenoliccompounds. This regeneration capability, combined with their flexible alkyl chain that facilitates easier hydrogen transfer, makes phenyl propionates the most effective class in this study.
The computational results align well with the experimental results, showing that it is possible to predict properties using DFT calculations and to identify promising candidates for future studies. Furthermore, it is possible to predict the most likely mechanisms by which the antioxidants will react, allowing for explanations of unexpected behavior in the DPPH assay.