Understanding and Exploiting an Iron Catalysed Route to New Chemical Synthons II
Introduction
The project focuses on an iron catalysed method to prepare unprecedented chemical synthons, developed in the group of Dr. Webster (University of Bath). The aim is to explore the catalytic cycle with computational means by the use of quantum chemical calculations. This involves both, the identification of key intermediates and transition structures en route to product formation, as well as investigation of potential catalytically active transition metal species itself. The exploration of the catalytic cycle comprises the identification of key intermediates and transition structures using quantum chemical methods based on density functional theory. Careful validation of these methods through correlated ab initio theory, such as coupled cluster ansaetze, are applied for critical species, particularly with regard to electronic, thermochemical and kinetic properties. The resulting energy profile is compared with thermodynamic and kinetic observations from experiment. Furthermore, quantum chemical calculations allow to support the interpretation of experimental data as well as to suggest modifications that would lead to better performance in terms of catalytic activity and scope of applications.
Methods
This project relies on the quantum chemical description of transition metal systems featuring complex electronic structures with multiple low lying spin states. This involves highly correlated ab initio theory for a reliable benchmark and for the correct prediction of molecular electronic ground states as well as the relative energies of corresponding electromers and isomers. Already the calculation of a single species alone requires substantial amounts of resident memory of the order of several hundred GiB and a few TiB of disk space. Quantum chemical calculations were conducted employing density functional theory (DFT) and correlated coupled cluster ab initio theory (DLPNO-CCSD(T)) with extrapolations to the complete domain space and basis set limits.
Results
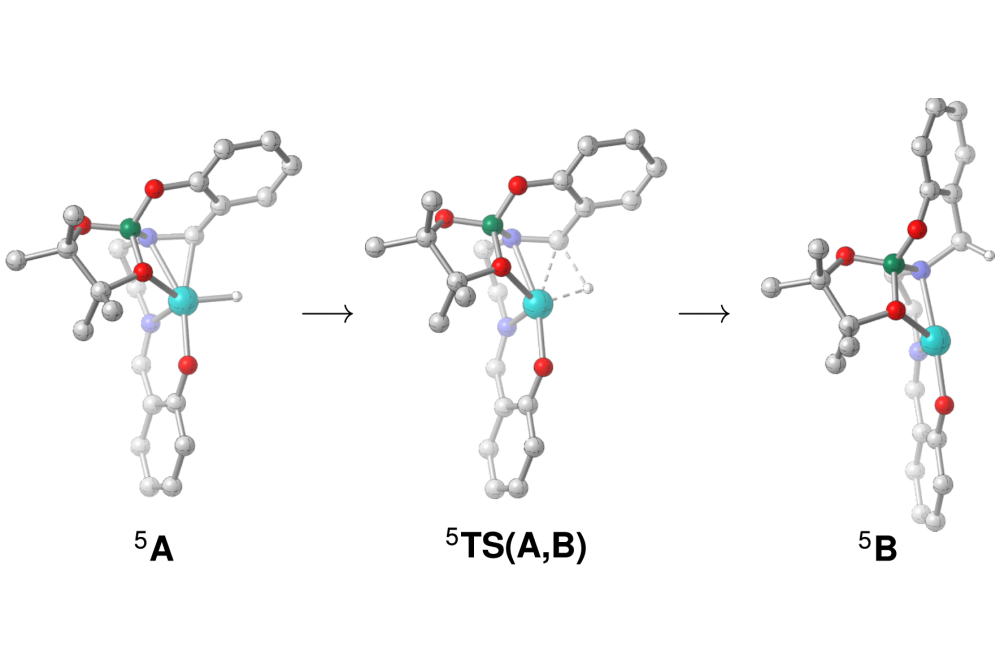
In the experimental lab an iron(salen) precatalyst was prepared to study its activation during alkyne cyclotrimerisation. A range of spectroscopic techniques provided evidence for the nature of the iron catalyst. These spectroscopic observations infer ligand reduction from an iron-boryl salen towards an iron-boryl salan complex as the key on-cycle intermediate.
Discussion
DFT studies have been used to interrogate spin states and low energy isomers of the on-cycle iron-boryl species. Correlated coupled cluster calculations identify the iron-boryl salen complex as a high spin species, which can transform via a 1,2 hydrogen shift reaction towards ligand reduction (see Figure). This finding triggered the detailed study of a homologous series of iron(salen) complexes, which shows that the air stable iron(salen) precatalyst is activated via ligand reduction. The resulting iron-boryl(salan) acts as highly sensitive and highly active catalyst for alkyne cyclotrimerisation.