Investigation of Spin Isomers in SnNFe with N = 8-16
Introduction
In the framework of the Collaborative Research Center (CRC 1487), iron is studied as a substitute for rare-earth metals in environmental-friendly technologies, since the rare-earth metals are toxic or obtained by disputable methods. For this, it is required to investigate iron in different environment in order to reveal its characteristics. Small doped tin clusters are very suitable systems because their behavior are often tunable by the number of tin atoms. We have already investigated neutral SnNFe clusters with N = 8-16 with a spin multiplicity of S = 2 (quintet) in order to compare the quantum chemical results with experimental data. However, multireference calculation of the Sn8Fe from another group at the TU Darmstadt suggest, that this cluster species possesses a spin multiplicity of S = 1 (triplet). Therefore, we wanted to investigate the possibility of different spin multiplicities for all possible structural candidates generated by a genetic algorithm [2]. Based on that, a deeper understanding of the magnetic and electric interplay between the diamagnetic host cage and the paramagnetic dopant is obtained.
Methods
From a pool of structural candidates generated by a genetic algorithm developed in our group [2] geometrical isomers in the range of 1 eV relative to the energy of the energetically lowest-lying geometry are optimized on the PBE0/def2-TZVPP level of theory. The existence of local minima has been verified by frequency analysis. These calculations were carried out with the quantum-chemical program Gaussian16. For some cluster sizes the geometry optimization were also carried out with ORCA 5.0.4 to compare the results and rule out influences from the codes used to calculate the final geometric structures and energies.
Results
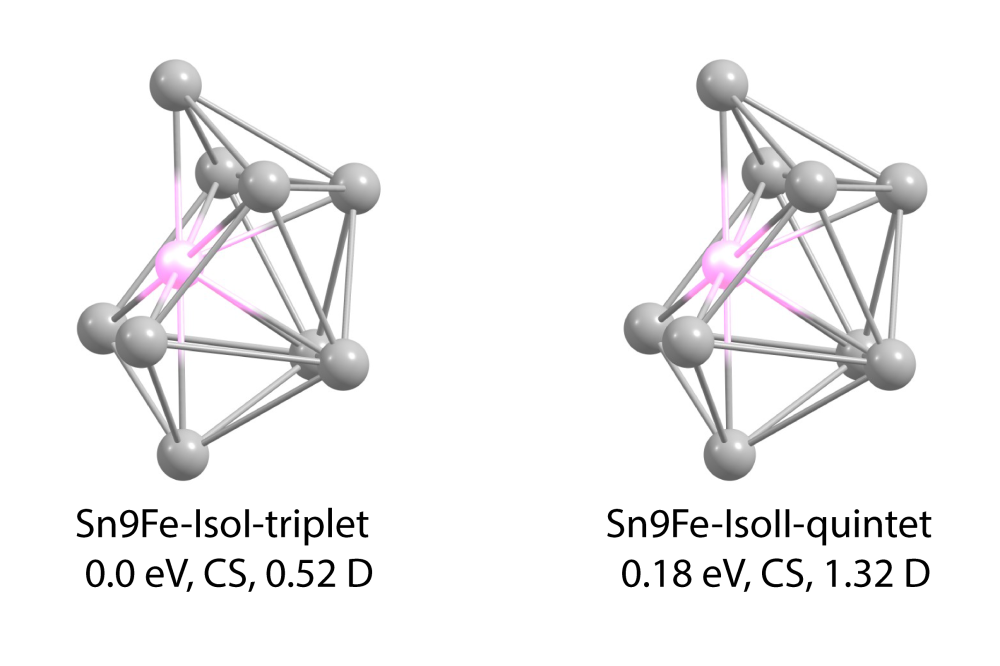
At first, the structural candidates of Sn11Fe cluster were identified with multiplicities of S = 1 (triplet), 3 (septet) and 4 (nonet) (S = 2 was already identified in the previous project [2]) and their energies were compared to the value of the global minimum (GM). Here, we could observe that the energy of the calculated geometries depend strongly on the spin multiplicity, resulting in energies differences up to 3 eV relative to the GM for the highest multiplicity, making these spin isomers the most improbable structures to be present in the experiment. The septet isomers also showed very high energies of up to 2 eV with respect to the GM. None of the calculated candidates with nonet and septet multiplicity was found in the energy range of 0.2 eV which is considered to be relevant in comparison to our experiment. However, the calculations of the triplet isomers showed similar and sometimes even lower energies compared to the quintet, confirming the results of the multireference calculations on the Sn8Fe cluster. Based on this observation, we systematically studied the triplet isomers for the SnNFe clusters with N = 8-16.
Discussion
By investigating the triplet isomers of the clusters mentioned above, at least one triplet isomer was found in the energy range of 0.2 eV compared to the GM for all considered cluster sizes except for the Sn12Fe. In case of the clusters with N = 8-10 and 13, the triplet isomers are the new GM. For some cluster sizes, the quintet and triplet isomers posses very similar geometric structures. However, the dielectric properties are considerably different. Measuring the electric dipole moment allows then not only to discriminate the geometric structure but also the spin multiplicity of the cluster.