Density Functional Theory: Study on the Mechanism of the Chloride-Induced Aufbau of Perchlorinated Cyclohexasilanes
Introduction
Nano- and micrometer-scaled silicon structures are essential components not only in microelectronic but also in photovoltaic and opto-electronic applications. Oligo- and polymeric (perchloro)silanes are suitable precursors for the formation of silicon wires or thin silicon fi lms.
Methods
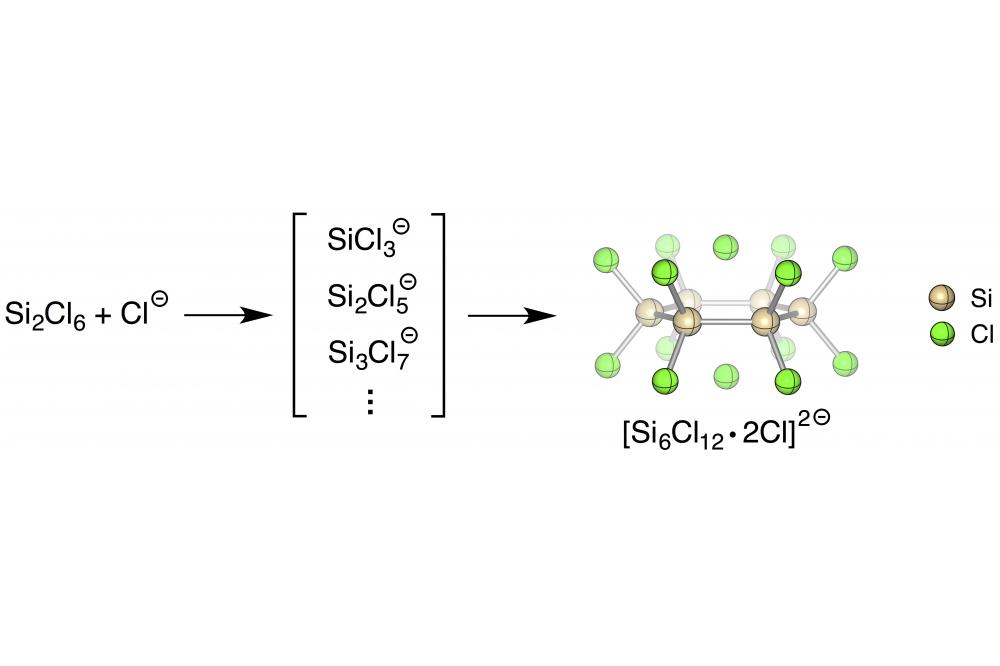
The mechanism of the amine-induced disproportionation of perchlorinated silanes aff ording neo-Si5Cl12 was recently established.[1,2] A surprisingly simple preparative procedure, the addition of Si2Cl6 to a solution of [nBu4N]Cl in dichloromethane, leads to the formation of a chloride-complexed cyclic dianion [Si6Cl12∙ 2Cl]2- and a variety of its silyl-substituted structural analogs, depending on the reaction conditions. The underlying reaction mechanism has been elucidated by DFT calculations (Fig. 1).
Results
It reveals the chloride ion itself as a Lewis base to trigger a disproportionation of perchlorinated silanes with a subsequent buildup of dianions containing up to eight silicon atoms.[3]
Outlook
The mechanistic insights gained provide the fundament required for the targeted synthesis of oligosilane precursors suitable for microelectronic applications.