Theoretical Investigation of Electrocyclic Reactions of Siloles
Introduction
Siloles are five-membered heterocyclic dienes, in which a silicon atom replaces one of the carbon atoms of cyclopentadiene. Silole derivatives find application as building units for σ- and π-conjugated compounds for use in organic electroluminescent devices,[1] and their synthesis, reactions, and properties have been subject to intensive studies.[2]
Results
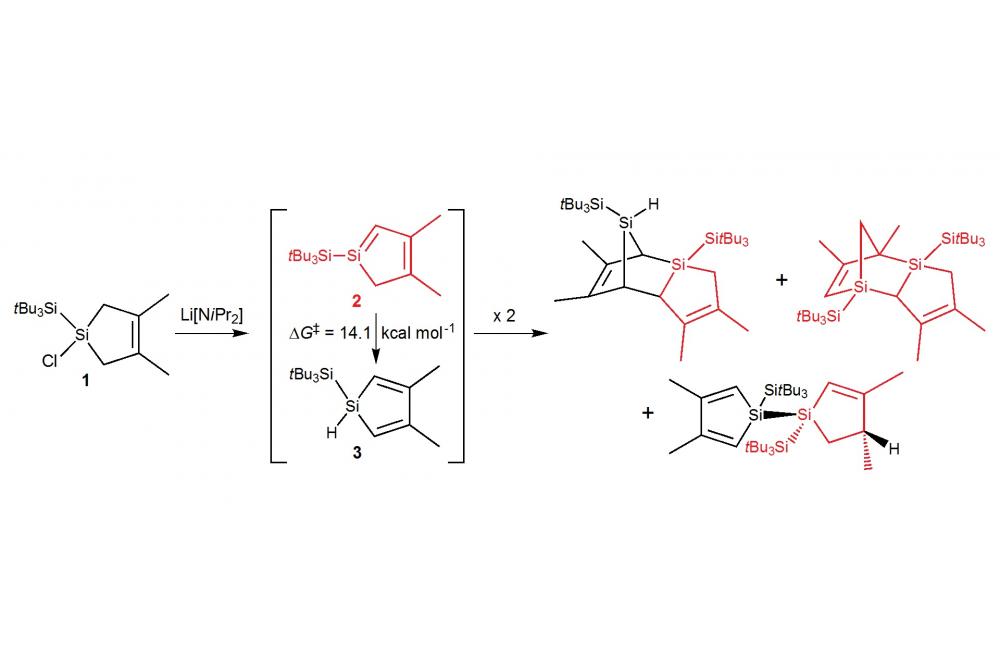
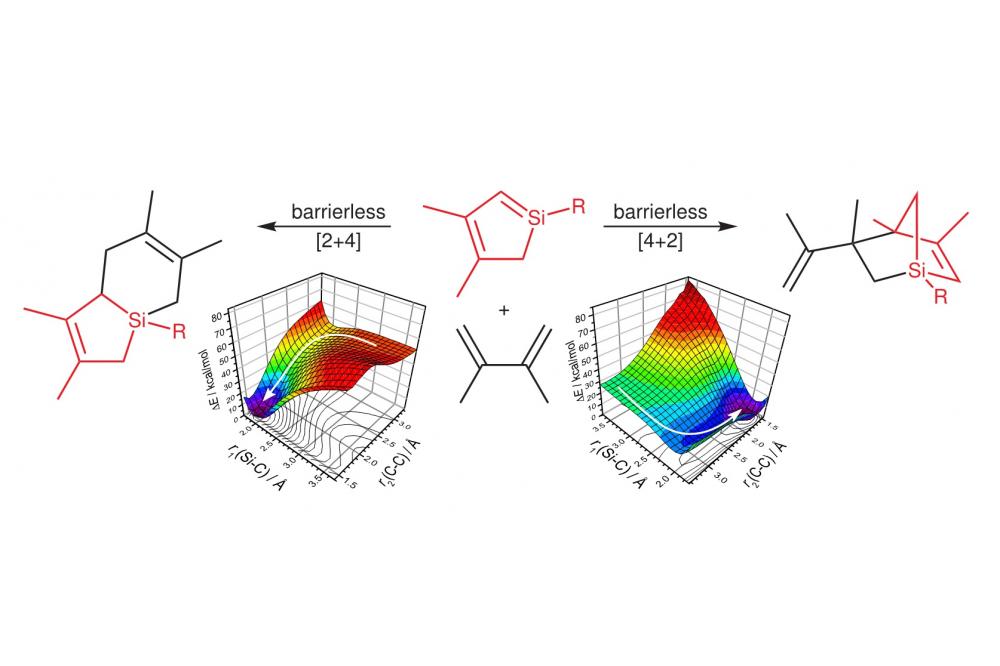
In an earlier investigation, the 1-chlorosilacyclopentene 1 has been obtained starting from perchlorinated silanes, Li[SitBu3], and 2,3-dimethylbutadiene[3] and siloles can readily be obtained from this compound by deprotonation and LiCl elimination. In a recent combined experimental and theoretical study we have shown that the resulting siloles are very reactive and can isomerise, dimerise or be trapped by reagents like cyclohexene or 2,3-dimethylbutadiene, and the mechanisms underlying these reactions have been investigated in detail by means of double-hybrid density functional theory calculations.[4] Silole 2 can easily isomerise to the more stable silole 3 (Figure 1) via hydrogen shift. Alternatively, it can also dimerise to yield several products: two dimers are formed via Diels-Alder reactions (2+3 and 2+2) and the last one is an head-to-head dimer of 2 and its isomer 3. This latter, more unusual product is accessible via both singlet and triplet intermediates (Figure 1). Further, compound 2 can be trapped prior to isomerisation or dimerisation by addition of excess cyclohexene yielding the racemate of the [4+2] cycloadduct. Under similar conditions, the reaction of 2 with 2,3-dimethylbutadiene yields the [2+4] and [4+2] cycloadducts and hence, 2 acts as silene (dienophile) as well as diene. Our results show that both reaction steps occur without activation barriers (Figure 2).