Well-Defined Iron Catalysts for the Acceptorless Reversible Dehydrogenation-Hydrogenation of Alcohols and Ketones
Introduction
The oxidation of alcohols to esters and the reverse reaction (reduction of esters to alcohols) is an important chemical transformation frequently used in industry and academia. The two classical protocols for the reduction of esters are the use of stoichiometric amounts of a hydride reagent on the one side and the catalytic heterogeneous hydrogenation on the other side. Both processes come along with substantial drawbacks, i.e., the toxicity of the reagents in the former case and the poor chemoselectivities for heterogeneous catalysts. To perform the desired reaction under milder conditions, the development of homogeneous catalysts is a promising approach. However, most homogeneous catalysts are based on ruthenium as the active transition metal, which poses environmental concerns.
In the year 2014, several working groups independently development iron complexes for the transformation from alcohols to esters (see structures 1 – 3). The use of an earth-abundant and non-toxic metal in these systems, combined with high catalytic activities and chemoselectivities, clarify the possible great impact of the iron catalysts in both industry and academia [1-3] (see reference [4] for a detailed review on iron catalyzed dehydrogenation reactions).
Methods
In cooperation with the groups of Schneider (Goettingen University), Hazari (Yale University), Bernskoetter (Brown University) and Jones (University of Rochester) a combined experimental and density functional theory (DFT) study was performed to investigate the substrate scope for the alcohol dehydrogenation and to gain deeper insight into mechanistic details of the catalytic cycle [3].
The computational investigation of the mechanistic details was performed on a model system for the iron (II) catalyst and several plausible reaction pathways for the dehydrogenation of methanol were studied. In the molecular model system, the iPr2 groups were replaced by Me2 groups to save computational resources.
Results
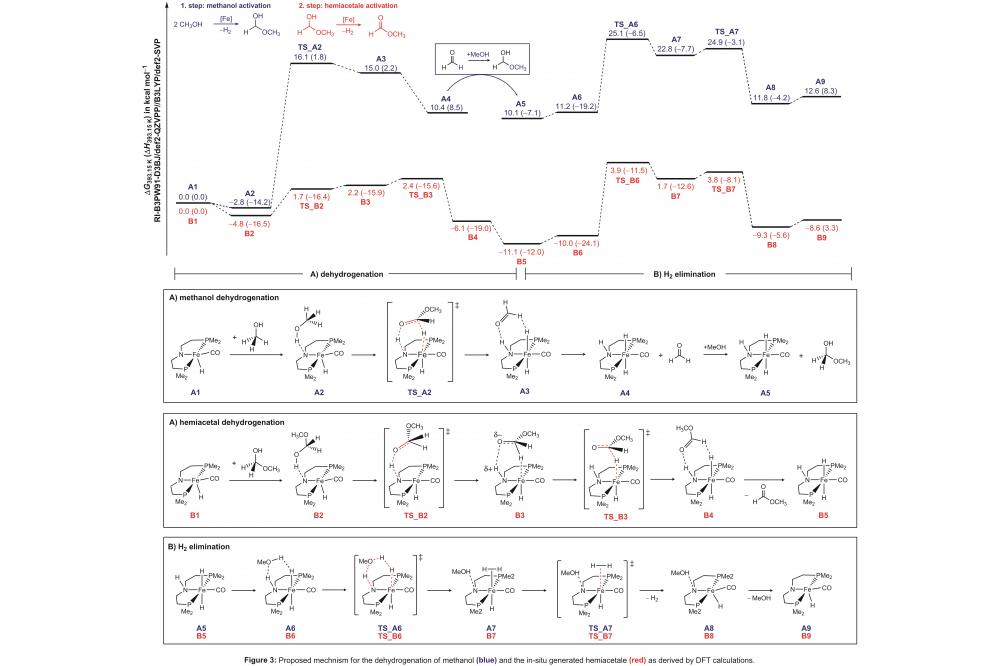
The resulting catalytic cycle, as depicted in figure 3, is composed of two seperate parts: the dehydrogenation of methanol (blue) and the dehydrogenation of the hemiacetale formed in-situ (red). The computations revealed a moderate activation barrier of 16.1 kcal/mol for the dehydrogenation of methanol (TS_A2, see figure 3). Subsequent elimination of H2 occurs with an activation barrier of 25.1 kcal/mol (TS_A6, see Figure 3). In this step, metal-ligand cooperativity of the catalyst plays a crucial role. The second part of the reaction pathway (red) is combined with a significantly lower overall activation barrier of 15.0 kcal/mol (TS_B6). The overall rate-limiting step of the catalytic cycle is given be the H2 elimination from complex A5. Dihydrogen is liberated by a methanol-assisted proton shuffle mechanism (TS_A6) with a barrier height of 25.1 kcal/mol.
Outlook
Further improvement of the employed catalysts is currently under investigation. As a part of this work, the employed DFT methods are evaluated by comparison to high-level coupled cluster results.