Measuring London Dispersion Interactions in Solution
Introduction
Adding dispersion energy donors (DED) to a system can severely change the outcome of a reaction.[1] The concept of a DED has only been established in recent years[2], and since, several systems to quantify London dispersion in solution have been developed. However, many of them contain heteroatoms, which are able to participate in hydrogen bonding or dipolar interactions, making it difficult to differentiate between LD and other noncovalent interactions.[3]
Methods
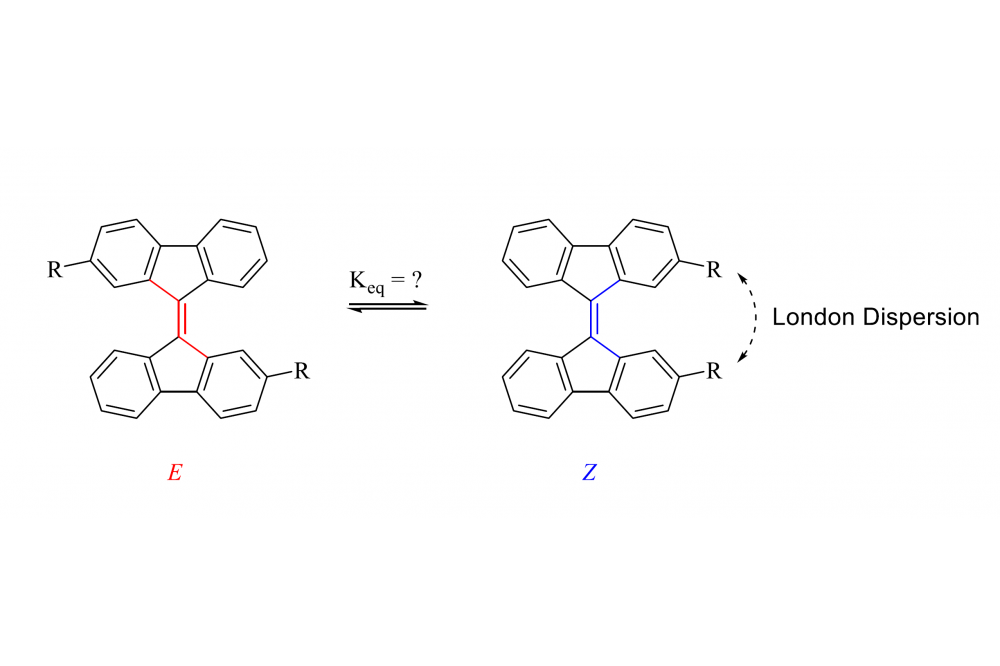
We are synthesizing 2,2’-substituted 9,9’-bifluorenylidenes as hydrocarbon based dispersion balances. This system is easy to synthesize and, since it is based on E/Z isomerization, can be equilibrated thermally as well as photochemically. ΔGE/Z values are obtained from NMR-data and used to understand how strongly the different substituents influence the position of the equilibrium state. As substituents we are using linear, cyclic and cage-type hydrocarbons. Weare performing DFT computations of our system to compare the obtained computational data with our experimental results.
Results
First results show that the Z-isomer is generally preferred, supporting our working hypothesis that the observed equilibrium is mainly influenced by LD. This trend can also be seen for the computational data.
Outlook
Solvent- and temperature dependent measurements of the E/Z equilibrium of several different 2,2-substituted 9,9’-Bifluorenylidenes will be carried out soon. This allows us to get a good insight in how London dispersion interactions behave in solution.