Activation of Small Molecules With Metal-Free Systems
Introduction
Ammonia borane (NH3BH3) is an easy to handle solid capable of releasing up to 3 equivalents of dihydrogen and therefore a potential material for energy storage applications. For the release of dihydrogen at mild conditions accessible and cheap catalysts are highly desirable.
Methods
Catalyst design and corresponding mechanistic investigations were supported using highlevel quantum mechanical computations.
Results
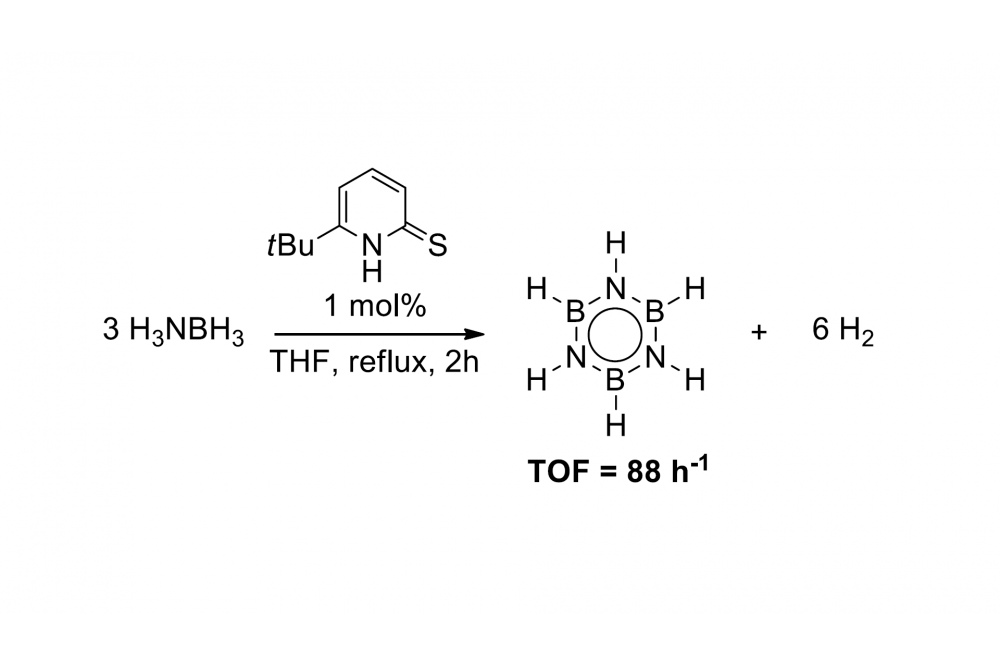
We reported the first example of an organocatalyst capable of releasing dihydrogen from ammonia borane at mild conditions which shows comparable activity to noble metal based catalysts (scheme 1).[1] The high level computations helped extensively in elucidating the reaction mechanism of the dehydrogenation of ammonia borane using 6-tert-butyl-2-thiopyridone as catalyst.
Discussion
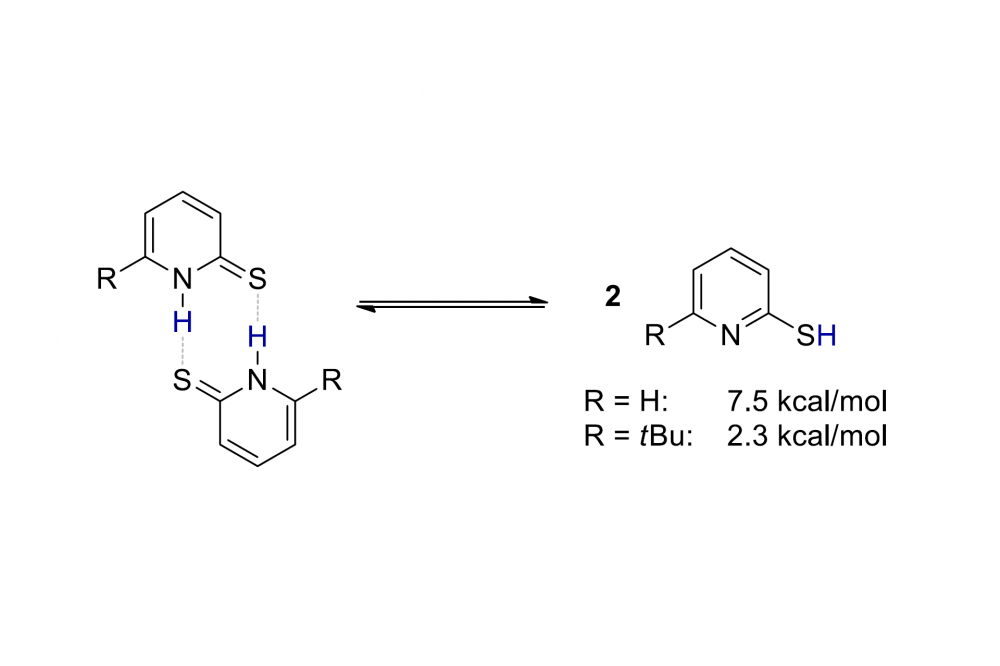
Computations revealed that the comparable high catalytic activity can be partly attributed to the tert-butyl moiety of the catalyst. The catalyst exists in solution in dimeric form. To activate ammonia borane it has to monomerize which is significantly easier when the tert-butyl group is present destabilizing the dimeric form because of the steric bulkiness (scheme 2).
Outlook
Using high level quantum mechanical computations we aim to develop new efficient catalyst for the activation of small molecules either for the release of dihydrogen or the activation of dihydrogen for further chemical transformations.