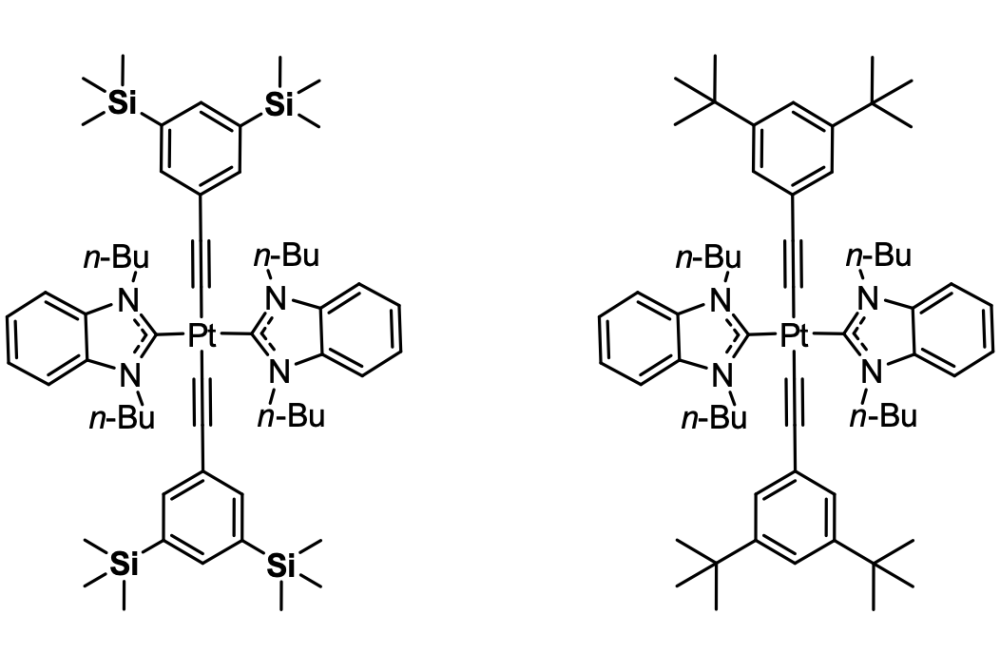
Controlling the Aggregation of Phosphorescent alkynyl-Pt-NHC Complexes via Silyl-DED Substitution
Introduction
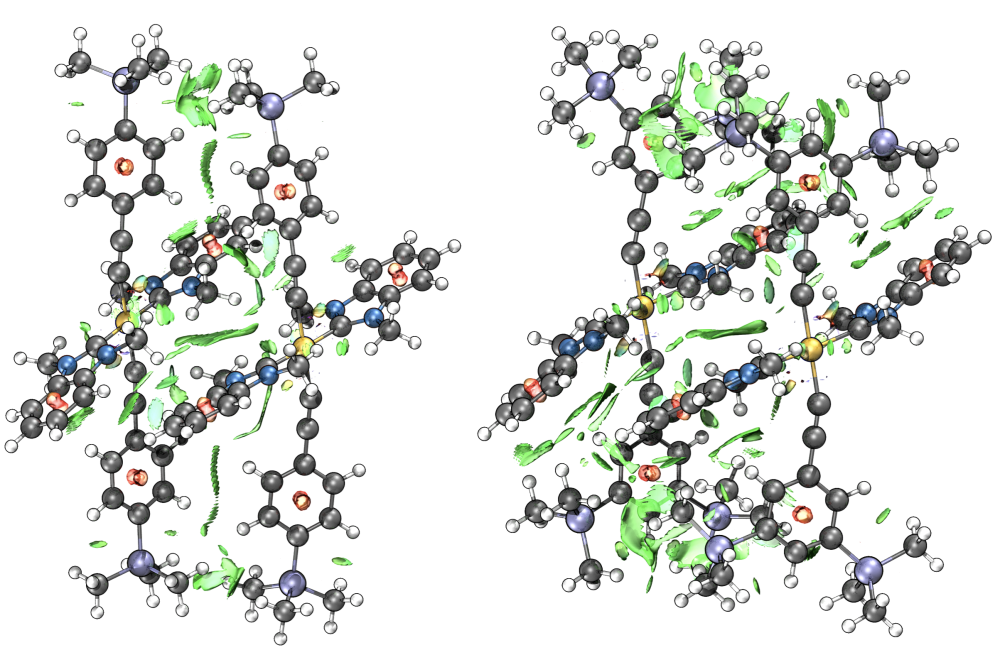
Non-covalent interactions (NCIs) not only govern the structure biomacromolecules such as proteins and DNA, but often play a key role in the outcome of chemical reactions. Amongst NCIs, the Van-der-Waals forces, London dispersion (LD) forces or induced dipole-induced dipole interactions are the weakest and therefore often underestimated. However, since LD interactions are approximately additive pairwise interactions, large (alkyl and silyl) groups must be more than just providers of steric bulk. While LD interactions between alkyl groups are capable to facilitate allegedly labile compounds such as hexaphenylethane or coupled diamondoids by offering intramolecular stabilization, the effects of commonly utilized silyl groups have not been studied in great detail.
The incorporation of bulky silyl groups, in particular trimethylsilyl (TMS) groups, in luminescent/phosphorescent systems has shown to improve the optophysical properties (color purity, quantum yield) of pyrene based fluorescent and Pt-N-heterocyclic-carbene (Pt-NHC) based phosphorescent emitters. This effect is usually attributed to the greater bulkiness of silyl-DEDs in comparison to carbon-DEDs, which results in a reduced aggregation tendency, and hence a lower non-radiative decay rate due to triplet-triplet annihilation. However, silyl-DEDs and carbon-DEDs are approximately similar in size, and silyl-DEDs even exhibit a greater polarizability, which should lead to stronger LD interactions in aggregates. This raises the question of whether the considerably different aggregation behavior, and hence the difference in the photophysical properties of silyl- and carbon-DED substituted emitters can solely be traced back to the miniscule differences in in steric bulk?
Methods
We use TD-DFT computations to simulate the absorption and emission properties of potential emitters. The conformer ensemble for each compound is analyzed via semiempirical methods. Together, this allows us to create weighted optical spectra to assess the feasibility of each compound to be used in potential OLED materials.
Results
Recently, a class of phosphorescent trans-alkynyl Pt-NHC complexes have attracted considerable attention due to their deep-blue phosphorescent emission and their electroluminescence (EL) properties, making them attractive targets for potential OLED applications. These complexes however, suffer from low quantum yields at doping concentrations typically found in OLED devices as a result of aggregation. Our experimental results and some initial computations point towards a combination of electronic effects and a significant dispersion-dominated change in aggregation behavior (Figure 1).
Discussion
We expect to gain additional insight on the latter effect by carrying out DFT/semiempirical conformational analyses on various phosphorescent Pt-NHC complexes carrying bulky -tBu and TMS groups (Figure 2) and comparing computed thermochemical and photophysical data with experimentally obtained phosphorescence quenching data. This will allow us to investigate why silyl- and carbon-DED substituted compounds behave so differently, and to predict how silyl-DED-functionalization can be utilized most efficiently for controlling the aggregation behavior of this class of phosphorescent alkynyl-Pt-NHC complexes.