Design and Synthesis of Phosphinidene Chalcogenides
Introduction
Phosphinidene chalcogenides (R–P=E; Ch = O, S, Se) are phosphorus analogues of organic nitroso compounds (R–N=O). In contrast to the robust stability of R–N=O, phosphinidene chalcogenides have been considered extremely reactive species.[1-2] These transient species are very reactive toward organic molecules and have been thus used as efficient synthons for new organophosphorus derivatives.[3-5]
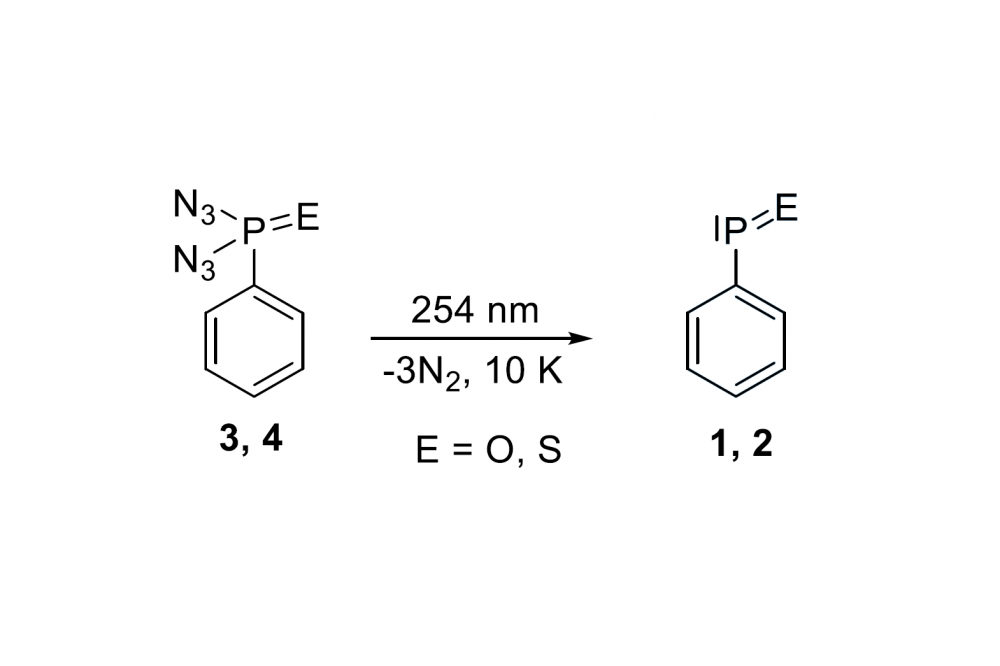
Stabilization of R–P=E species can be achieved through coordination to metal centers (thermodynamic stability) or using bulky substituents (kinetic stability). Phenylphosphinidene oxide Ph–P=O (1) (the phosphorus analogue of nitrosobenzene) and phenylthiooxophosphine Ph–P=S (2), are hitherto unknown experimentally, although the structures and energies of Ph–P=E (E = O, S) and their metal complexes have been computationally and experimentally studied.[6]
Discussion
Herein, we aim at synthesizing and isolating yet unreported Ph–P=O and Ph–P=S through either flash vacuum pyrolysis or photolysis of their respective azide precursors PhP(E)(N3)2 (E = O, S), thus enabling further studies on their structures and reactivities as ligand-free species. We plan to extend our method to exploit mono-substituted azide precursors. Similar azide architectures have allowed a facilegeneration of transient nitrenes upon irradiation or themolysis.[7]
Outlook
We will study their chemical and physicalproperties using both experimental and computational methods. Density functional theory (DFT) and ab initio computations will be used to determine the substituent effects on the vibrational as well as optical spectra, photochemistry, geometries, and transitions states for subsequent reactions.