Prebiotic Formation of Sugars and Amino Acids Using Aminomethylene
Introduction
After the matrix isolation of aminomethylene (H-C̈-NH2)[1] we study further the formation of amino acids and sugars in quantitative flow pyrolysis experiments with cyclopropylamine asstarting material. After pyrolysis at 900 °C aminomethylene is generated by ethylene extrusionand undergoes [1,2] H-shift to methanimine. During the pyrolysis a huge amount of HCN is released by the decomposition of aminomethyle or methanimine which reacts with methanimine to aminoacetonitrile during warm-up of the cold trap. Hydrolysis with conc. HCl should lead to the amino acid glycine according to Strecker’s synthesis. By rinsing the cold trap with water imine can also be hydrolyzed to formaldehyde which immediately forms glycolonitrile with HCN in solution. Computations are used as a helping tool to investigate these reactions.
Methods
For computational studies, the program gaussian16 is used. For geometry preoptimizations we use hybrid functionals as B3LYP with high basis sets. For accurate computations we used coupled cluster methods.
Results
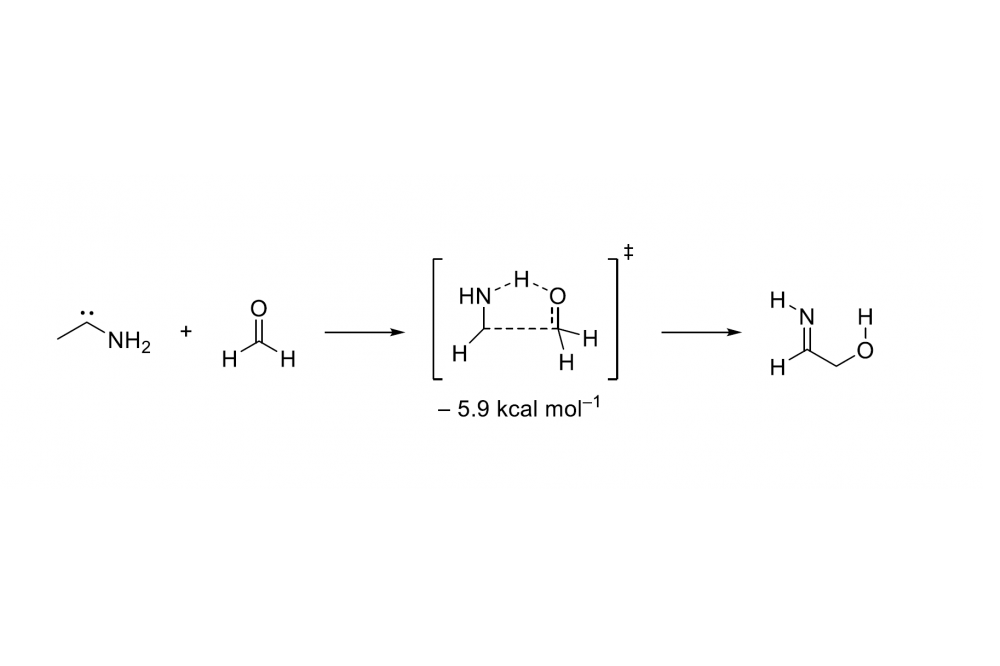
We were able to identify aminoacetonitril and glyconitrile via NMR spectroscopy and GC-MS in the condensed residue of flow pyrolysis experiments which confirm our working hypothesis. We were not able to identify formaldehyde in the spectra suggesting that the subsequent reaction with HCN is very fast. The high level ab initio computations indicate that the reaction is almost barrierless as for hydroxymethylene and formaldehyde. Formaldehyde and aminomethylene form a prereactive complex of −6.3 kcal mol−1. Over a small barrier of 0.4 kcal mol−1 that corresponds to a rotation of formaldehyde in the plane of aminomethylene it undergoes to iminoethanol. The C-H-insertion goes over an unfavored barrier of 21.3 kcal mol−1 to aminoacetladehyde.
Outlook
Based on these results, we continue the experiments that we can show the simultaneous formation of sugars and amino acids. Hereby, aminomethylene can further react in a barrierless carbonyl-ene reaction with formaldehyde to give iminoethanol, which then can be further converted to serinonitrile or be hydrolysed to glycolaldehyde.