Synthesis and Conformational Analysis of Parent Perhydroazulenes
Introduction
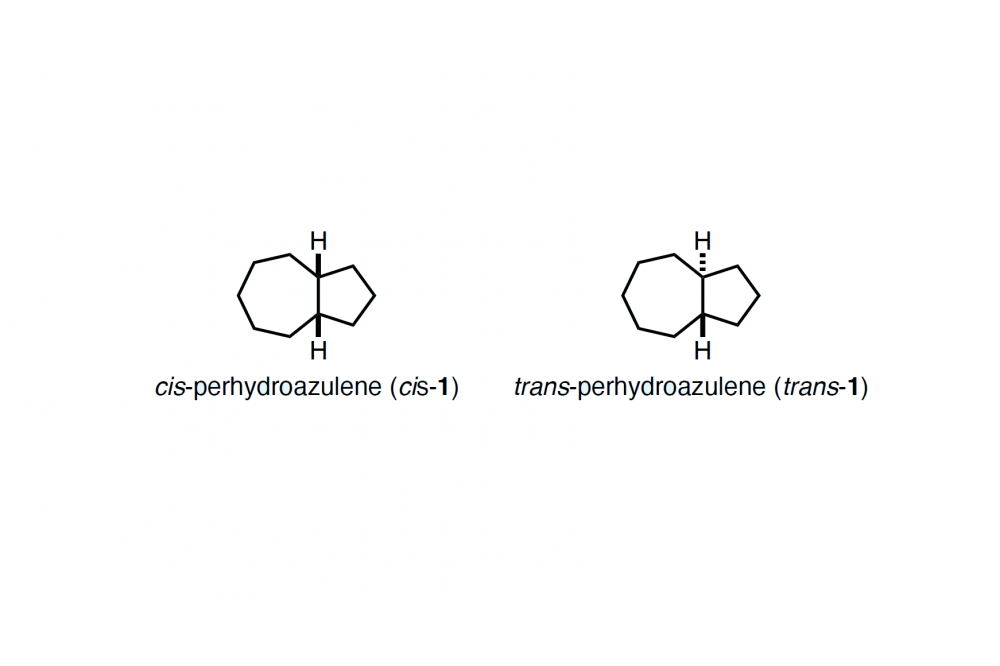
Perhydroazulenes are common structural motifs in a variety of terpene natural products. Despite of their structural importance, studies on parent perhydroazulenes, cis- and trans-1 (Fig. 1), have been scarce. In this project, we aim to establish short synthetic routes to both diastereomers cis- and trans-1 and carry out their thorough conformational analysis to study the relative stability between them.
Methods
The project consists of experimental and computational parts. In the experimental part, we synthesize the two diastereomers cis- and trans-1 through multistep organic synthesis using a cheap, readily available starting material. In the computational part, we perform density functional theory (DFT) and MP2 geometry optimizations and frequency computations for conformations of cis- and trans-1.The relevant conformers are subsequently subjected to single point energy computations using the coupled cluster method.
Results
The synthesis of cis-1 was carried out using cycloheptanone as a starting material. The key step enroute to cis-1 was the stereoselective (and chemoselective) reduction of α,β-unsaturated ketone to establish the cis ring fusion. On the other hand, the synthesis of trans-1 involved 1,4-addition of an organocopper reagent to the cycloheptenone starting material followed by electrophilic trapping of the enolate as the key stereoselective step. We obtained both cis- and trans-1 in diastereomerically pure form.
Next, we performed geometry optimizations and frequency calculations for conformations of cis- and trans-1 at the B3LYP, B3LYP-D3(BJ), M06-2X, B2PLYP, B2PLYP-D3 functionals and the MP2 method with a cc-pVTZ basis set. For the MP2/cc-pVTZ optimized structures, we also performed CCSD(T)/cc-pVTZ single point energy computations. These computations revealed that cis-1 is 0.7–1.0 kcal mol–1 more stable than trans-1.
Discussion
After careful analysis of steric and torsional strain in cis- and trans-1, we reason that an additional gauche interaction present in trans-1 accounts for the computed energy difference of 0.7–1.0 kcal mol–1. This result was unexpected and at the first sight, seemed contradictory to previously reported experimental data where the Pd/C-catalyzed isomerization between cis- and trans-1 at high temperature (550 K) resulted in a trans/cis ratio of 1.6. However, by taking the Boltzmann distribution into account, all DFT and MP2 computations listed in Methods provided a trans/cis ratio of ca. 2 (1.6 at the MP2/cc-pVTZ level of theory), thus an excellent agreement with the experimental data. We conclude that the most stable conformer of cis-1 is energetically lower-lying than that of trans-1, but at equilibrium at high temperature, the conformers of trans-1 become more populated than those of cis-1.