Asymmetric, Lewis-Acid Catalysed, Diels-Alder Reactions of Phthalazine
Introduction
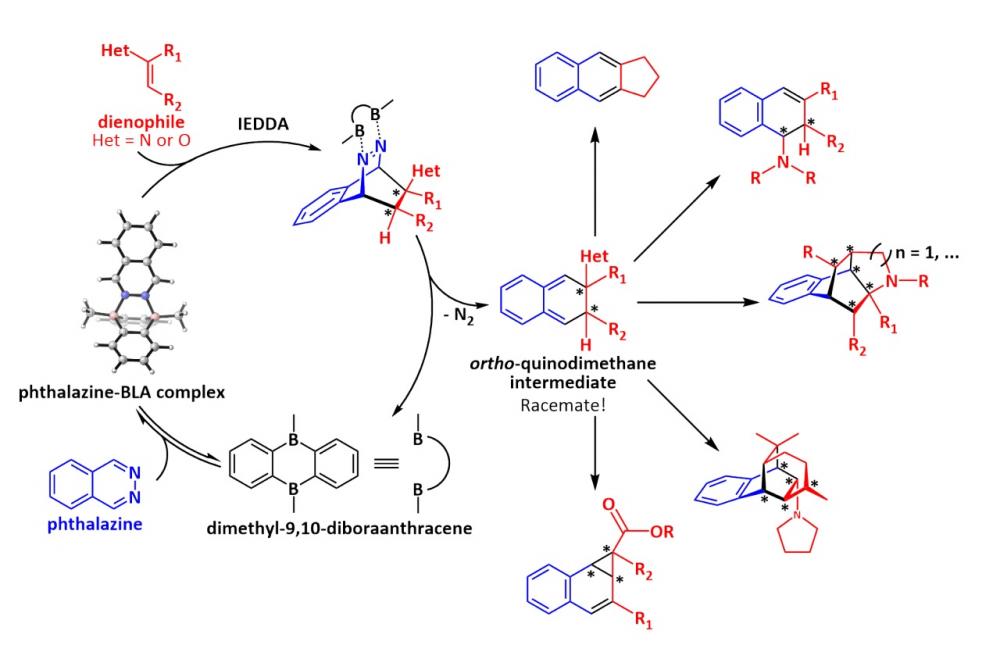
Novel methods to access complex molecular structures open new avenues for applications in medicalas well as material sciences. That is the reason why we investigate inverse electron-demand Diels-Alder (IEDDA) reactions of phthalazine. Starting from simple reactants like aldehydes, amines and phthalazine, complex structures can be generated within one step executing such IEDDA (Fig. 1). But phthalazine is an unreactive species in this context. Therefore, it has to be activated, for example by a Lewis-acid (LA) catalyst, like dimethyl-9,10-diboraanthracene (DBA). One major aspect of such reactions is the energy gap between the LUMO (lowest unoccupied molecule orbital) of phthalazineand the HOMO (highest occupied molecule orbital) of the dienophile. The orbital energies of phthalazine are lowered by bidentate coordination to the LA catalyst, which enables the reaction. Currently, we focus on the development of chiral bidentate LA to execute these transformations in an enantioselective fashion since chirality plays a crucial role. In this process, the 3D structure of the phthalazine-LA-catalyst complex is of particular interest.
Methods
Geometries can be modulated and energy gaps can be preestimated by computational chemistry. We design chiral LAs and apply computational chemistry to modulate the binding behaviour of phthalazineto these candidates. Sterics in addition to non-covalent interactions determine the favourable conformation of the resulting phthalazine-LA complex and control the orientation of the incoming dienophile. This information can be extracted from computations and be used to predict, which chiral LA candidates are promising, especially in the context of enantioselectivity.
Results
The most promising catalyst candidates are based on the 9,10-diboraanthracene scaffold, which is functionalized with various chiral substituents. We developed synthesis strategies to prepare them and already synthesised some of them.
Outlook
At the moment, we concentrate on the synthesis and characterisation of these bidentate LAs. In parallel we apply them to IEDDA reactions of phthalazine and investigate the enantiomeric ratios of the products.