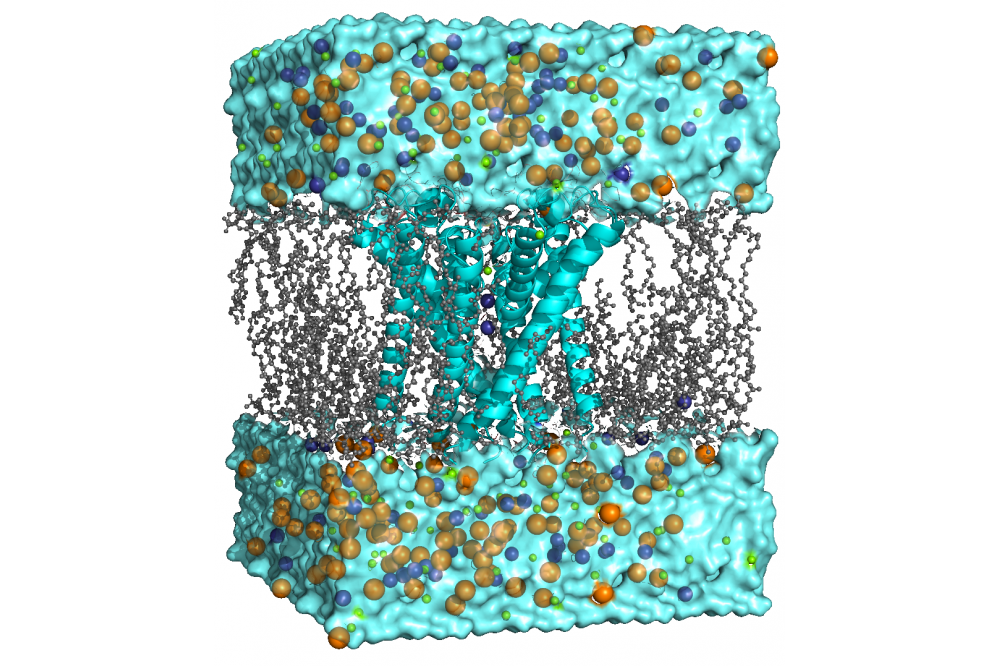
Molecular Dynamics Study of the Sodium/Potassium Channels HCN
Introduction
Ion channels play a fundamental key role in all living organisms and are crucial for the signal transduction of neurons in higher animals. The hyperpolarization-activated cyclic nucleotide-gated (HCN) family of sodium/potassium channels are members of this protein family that are characterized by slow and weakly potassium selective inward current at hyperpolarizing voltages. HCN channels are expressed in a broad set of tissues in mammalia and are involved in an equally broad range of biological processes: in sinoatrial node cells of the heart, they are molecular facilitators of the pacemaker current (also known as ”funny current” If or Ih) which is required for subsequent generation of action potentials and ultimately leads to the sustainment of the heart beat. Beside that, they also play an important role in the neuronal activity of brain cells. Correspondingly, mutations within HCN genes give rise to a broad set of diseases such as heart arrythmias and epilepsy.
Methods
We use molecular dynamics (MD) simulations of the membrane-embedded channel to investigate various aspects of gating and selectivity in HCN channels. With this technique, the movement of atoms and molecules within the simulated time frame is approximated using classical mechanics. Furthermore, we incorporate enhanced sampling techniques and free energy calculations to sample processes not accessible via classical MD simulations and develop novel analysis tools.
Results
During the first project period, we focused on the effects of naturally occurring mutations on the HCN1 channel structure and why specific mutations cause epilepsy. In combination with in-vitro experiments, we were able to show that a specific mutation inside the central pore of HCN1 can stop the channel from closing but also blocks ion conduction. In contrast, other mutations had a less severe effect on the channel. Therefore, we have been able to give a plausible explanation to the question why this specific mutation is linked to severe forms of epileptic encephalopathy in human. During the second period, we used the same techniques to investigate the structure-function relationship in HCN channels by introduction of rationally designed mutations. We were able to show that the HCN domain functionally links the cAMP- and voltage-sensing domain to the central pore and thus plays a crucial role for channel gating. During the next two periods, we investigated how ions bind to the selectivity filter of HCN4 and revealed a novel conduction mechanism for sodium/potassium conductance unique to this channel species. Finally, we began began studying the influence of other ions on HCN conductance to further our understanding in processes like ion channel blockage and inhibition.
Discussion
Knowledge of the relationship between channel structures and experimentally determined features such as selectivity and conductance is critical for the development of remedies for HCN-related diseases while preventing off-target effects with potential side effects. Furthermore, a general understanding of complex ion channels is important for the development of tailored ion channels with pre-defined characteristics. These channels can have a broad set of applications in the future, i.e. as biosensors. Hereby, our MD simulation proved itself to be a valuable tool to bridge the gap between static structures obtained from structural biologists and electrophysiological measurements. In the future, we plan to further our understanding of several key aspects of HCN channels including voltage-dependent gating and especially ion conduction and selectivity mechanisms.