Atomistic Modelling of MAX and MXenes
Einleitung
Layered ternary compounds, known as MAX phases, represent a growing family of materials [1]. They are denoted by a general chemical formula Mn+1AXn, where M is a transitional metal (e.g., V, Sc, Ti), A represents a group A element (e.g., Al, Ga, Ge), and X is C and/or N. n = 1 − 3 (Figure 1). Apart from combined metallic and ceramic-like properties, they are precursors for two-dimensional (2D) MXenes (2D carbides/nitrides), which has increased surge in expanding the MAX family. Theory provides an efficient approach to discover novel compounds. Ab-initio high-throughput calculations coupled with thermodynamics have been widely employed for this purpose. Numerous studies, employing this approach, have predicted various MAX compositions [2, 3]. Dahlqvist et al. identified several MAX compounds by calculating formation enthalpies calculated relative to all competing phases [2]. A separate study predicted several additional compositions by utilizting total DFT energies of competing phases sourced directly from online density functional theory (DFT) databases (such as Materials Project (MP) [4]). This approach established a stability criterion of +120 meV/atom, which resulted in overpredictions. Here, in this study, we examine the stabilities of carbides across a wide range of MAX stoichiometries totaling 600. The compositional range encompasses all possible transition metals (M = Sc–Au), A = group IIIA–IVA elements, and X = C . We perform high-throughput DFT calculations on a high-performance computer to assess stability. The stability is evaluated by the formation enthalpy calculated against all known competing phases obtained from the MP and Open Quantum Materials Databases [5, 6]. The criterion for our thermodynamic (meta)stability is established at +30 meV/atom, based on the analysis of the thermodynamic stability in relation to experimental synthesizability. According to this criterion, we predict 68 new MAX compositions as stable (or metastable) out of a total of 600 compositions.
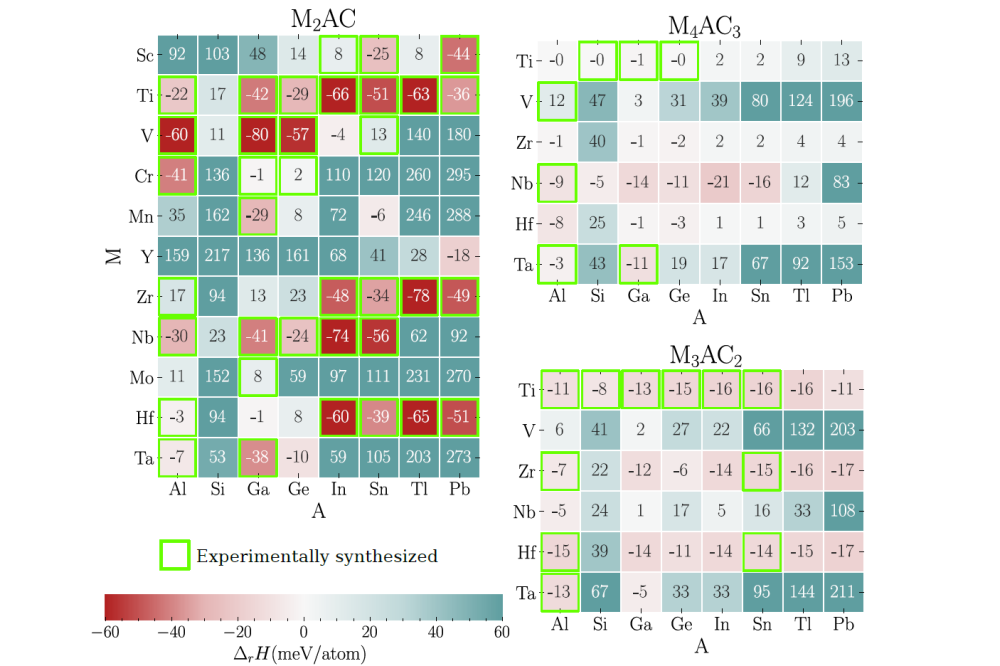
Methoden
The thermodynamic stability of a MAX phase is determined on the basis of the maximum formation enthalpy (ΔrH) relative to all (unary, binary, and ternary) competing phases (c.p), which is the difference of the DFT energy of a MAX phase compared to the most stable set of competing phases. The competing phases belong to a ternary M-A-X chemical system and are obtained from the online DFT databases, e.g., Materials Project [4], and open quantum database [6]. We find ΔrH as high as 17 meV/atom for the experimentally synthesized MAX phase, Zr2AlC (see the left panel in Figure 2). Such positive energy phases are classified as metastable. Therefore, we set our metastability criterion at +30 meV/atom, obtained by adding 10 meV/atom uncertainty error of the exchange-correlation functional used. Phases that have 0 < ΔrH < +30 are classified as metastable, whereas those have negative ΔrH are considered stable. The phases exhibiting ΔrH > +30 are considered unstable. Computational details are provided in [7].
Ergebnisse
Figure 2 shows the maximum formation enthalpies ΔrH of MAX carbides that are either stable (ΔrH < 0; red shading) or metastable (ΔrH < 30; light green shading). The experimentally synthesized MAX phases are outlined in a green frame. The medium and dark green regions indicate unstable compositions. Figure 2 left-top panel shows 35 stable and 15 metastable M2AC (n = 1) carbides. Out of these, 35 (30 stable + 5 metastable) phases have already been synthesized. Therefore, in total, we predict 5 stable and 10 metastable new M2AC compounds. From the bottom right panel of Figure 2, we find 25 stable and 10 metastable carbides of type M3AC2 (n = 2). Among these, 11 stable phases have already been synthesized. Hence, we add 14 stable and 10 metastable new M3AC2 compounds. The top right panel of Figure 2 shows 18 stable and 18 metastable M4AC3 (n = 3) phases. However, out of these, 6 stable and 1 metastable compounds have already been synthesized. As a result, we predict 12 stable and 17 metastable new M4AC3 phases. In summary, from the analysis of 600 compositions, we identify 68 new MAX carbides. Out of these carbides, 31 are stable, while the remaining phases are metastable.
Diskussion
Here, we performed high-throughput DFT calculations on 600 MAX compositions to evaluate their thermodynamic stabilities. The stability is assessed by the formation enthalpy (ΔrH) at 0 K calculated against all competing unary, binary and ternary phases present in a ternary M-A-X phase diagram. A metastability threshold of ΔrH < +30 meV/atom is set by the maximum value of ΔrH for experimentally synthesized MAX compounds. Based on this threshold, we predict 68 new MAX carbides as stable or metastable. The remaining MAX compositions are suggested as unstable.