Monte Carlo Modelling of Ion-Beam Cancer Therapy on Sub-Micron Scales
Einleitung
The modern radiation therapy of localized tumours applies accelerated beams of protons and carbon ions to deliver a high dose to the target volume while minimizing the dose to healthy tissues. This technique has the potential to reduce side effects compared to conventional radiotherapy using x-rays due to a better dose distribution in the patient. Despite of the successful clinical experience with proton and carbon-ion beams, other light nuclei can be also considered as possible therapy options. Questions can be raised whether light nuclei as helium could be more advantageous in particular cases.
Methoden
While a decisive answer should come from radiobiological experiments and clinical trials, theoretical simulations may help to identify optimal ion beams for cancer therapy, i.e. to evaluate the most appropriate ion to minimize the damage to healthy tissues at specific irradiation conditions. The Monte Carlo method is a powerful tool to describe the interactions of beam nuclei and all secondary particles with extended media. The Monte Carlo model for Heavy-Ion Therapy (MCHIT) [1] , based on the Geant4 toolkit,[2] was created in FIAS for simulating complex radiation fields in ion-beam cancer therapy.
MCHIT can be applied to simulate dose distributions in a tissue-like phantom representing a patient. It takes into account fragmentation of beam nuclei leading to secondary particles with their radiobiological properties different from primary nuclei. Besides, it is able to calculate the energy deposition in volumes of a few cubic micrometers. Such information is needed for modelling radiation effects in individual cells. MCHIT in combination with the modified Microdosimetric Kinetic model[3] has been applied to estimate the relative biological effectiveness (RBE) of different ion beams,[4,5] which is needed to scale the required x-ray dose to reach equivalent results for ions.
Ergebnisse
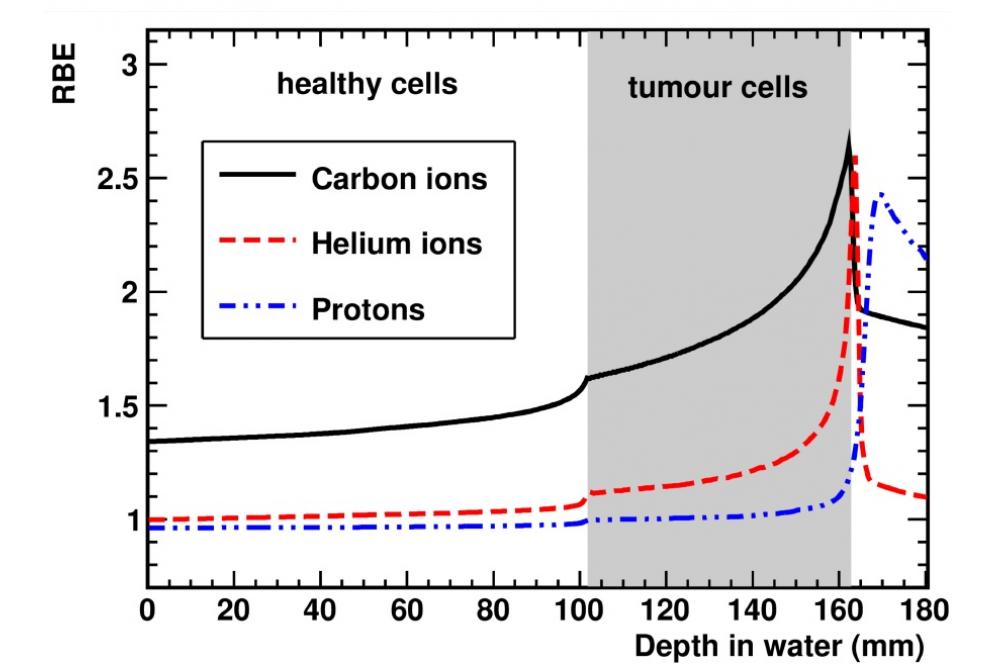
Figure 1 shows the predicted RBE in the case of a tumour located between 100 and 160 mm and surrounded by healthy tissues. As one can see, helium ions present an enhanced RBE at the depth of the tumour similar to carbon ions that helps to reduce the physical dose of the treatment. Besides, the RBE for helium ions in the healthy tissues located in front and behind the tumour is relatively low which helps to reduce the side effects in the healthy tissues. In addition, irradiation with helium ions lead to lower yields of secondary nuclear fragments and less dose at distal tissues compared to carbon ions.
Ausblick
Such results indicate that helium beam should be regarded as a promising option for ion-beam cancer therapy in the future.