Atomistic Computer Simulations of Amorphization in Metal/Metallic-Glass Multilayer Systems
Einleitung
Metals show a strong tendency to form crystalline, i.e., ordered phases. For unalloyed metals, the thermodynamically stable phase is always crystalline. Amorphous metal samples can be synthesized by „freezing“ them in the disordered state. This can be achieved for example by employing high cooling rates or depositing atoms with high energies or velocities. The resulting amorphous state is metastable. This metastable state can be made more favorable, although not stable, by alloying.
This material is then called a metallic glass. Apart from these amorphization processes driven by kinetic reasons, there are a few circumstances in which metals amorphize for energetic reasons. A well-known example is the interface between two different crystal phases: The order of the two phases is incompatible, making a sharp interface of two adjacent crystals energetically very unfavorable. The interface becomes disordered in a process called solid state amorphization (SSA).
Methoden
Due to their rarity, amorphous metals are interesting phases from a theoretical standpoint, and may well be useful for novel or improved practical applications. Amorphous glass/iron multilayer systems, e.g., are promising candidates for magentic tunnel junctions.[1] One recently discovered way of synthesizing such a system was developed by Ghafari et al.: By sputtering iron on a metallic glass substrate, they obtained a thin amorphous film of iron, as long as the film was not too thick.[2] Usually, iron will not be stable in an amorphous state, even if sputtered on an already disordered substrate. The question of interest is if the observed process is simply due to the conditions of the deposition process, or if the reasons for the amorphization of iron are energetic. To that end we conducted molecular dynamics computer simulations using the software LAMMPS. We chose a simple model system of a Cu-Zr based metallic glass with an embedded copper nanolayer. Just like iron, copper has a very strong tendency to form ordered phases.
Ergebnisse
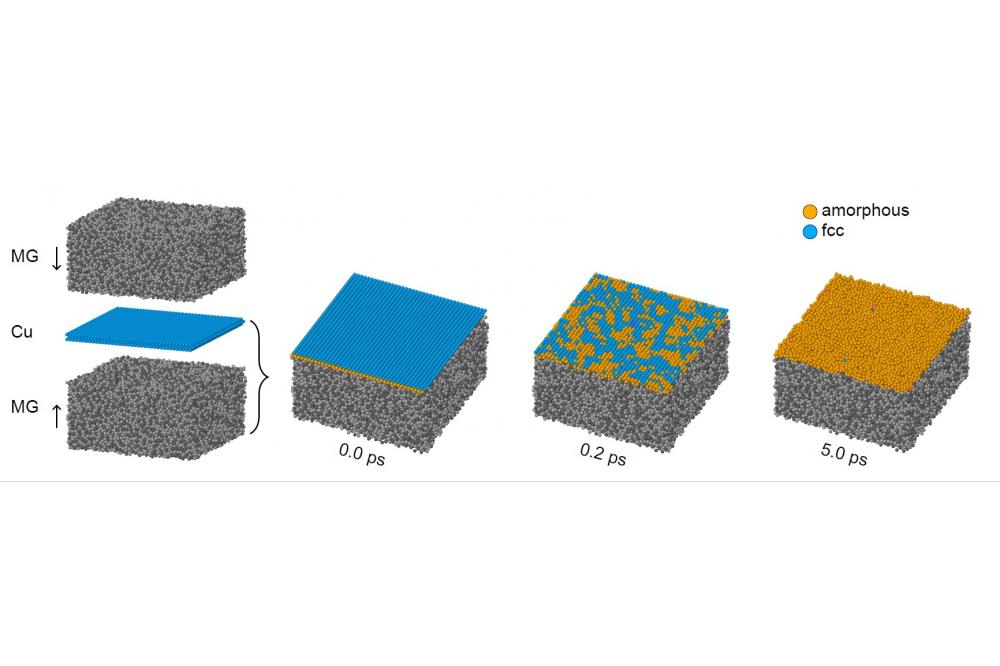
The simulation setup is shown in figure 1 on the left: We embedded crystalline copper nanolayers (Cu) of different thickness in the metallic glass (MG). By starting from a crystalline state, we can exclude that any transformation is due to deposition conditions that freeze the copper in an disordered state. Instead, any SSA-like effect must be due to energetic reasons. It can be seen in the three simulation snapshots on the right of Fig. 1, that a thin film of copper will indeed amorphize. We found acritical thickness of about 1 nm, above which the copper will stay crystalline. The order of magnitude of this number agrees with the experiments by Ghafari et al.[2] The reason for the phase transition is the interface energy: Despite the fact that there is no interface between two mismatched crystals, the glass–glass interface energy is lower than the crystal–glass interface energy. This can compensate the unfavorable amorphous copper phase up to a critical thickness.[3]
Ausblick
Using computer simulations, we could prove that the amorphization in the presented composite system is energetically driven. It stands to reason that this phenomenon also appears in different metallic multilayer systems. Due to the energetic nature of the transition, the synthesis of these systems should be easily achievable.