Insights Into Molecular Cluster Materials With Adamantane-Like Core Structure by Considering Dimer Interactions
Einleitung
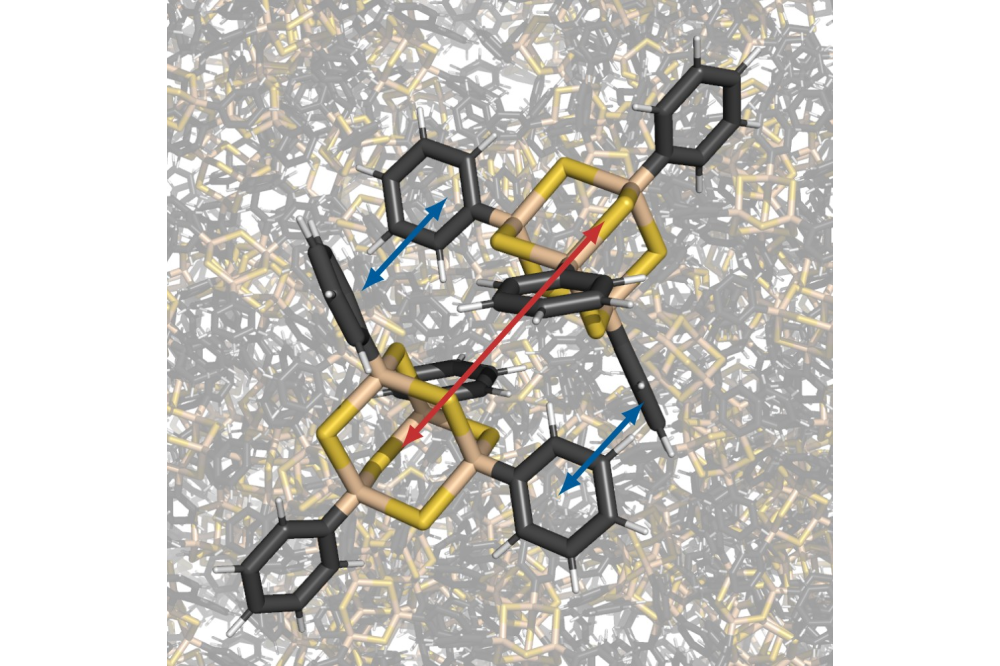
A class of cluster materials with an adamantane-like core structure (AdR4 or [(RE)4T6]; R=organic structure; E=Si, Ge, Sn; T=S, Se, Te) shows brilliant white light when irradiated with an infrared laser source.[1] This phenomenon occurs only in amorphous systems within this class of materials, while crystalline materials exhibit second harmonic generation. To gain insights into the molecular interactions within the materials, we performed a study on dimer model systems of 12 different chemical compounds in this class of materials.[2,3] Thereby, we investigated the binding characteristics, structural properties and polymorphs, and the scalability of the results.
Methoden
First, we performed a conformational search for each of the 12 compounds as monomer and dimer systems. Therefore, we employed the CREST program, developed by the Grimme group.[4] We performed this pre-optimization and conformational search at the GFN2-xTB level of theory. We subsequently optimized the structures at DFT B3LYP-D3(BJ)/cc-pVDZ(-PP) level of theory using Turbomole 7.3.[5] Finally, we carried out a single point calculations at B3LYP-D3(BJ)/cc-pVTZ(-PP) level of theory. The frequency calculations did not yield imaginary frequencies
Ergebnisse
The dimer structures were analysed in terms of the binding energy of the molecules to each other and their structural contributions.[2] This binding energy decomposition shows that the core-core interaction increases in the order: Ad<{Si4S6}<{Ge4S6}<{Sn4S6} and the substituent-substituent interaction in the order: methyl<phenyl<naphthyl. Both is reasonable, since the dispersive interaction is the dominant binding interaction in the materials considered. Of the 12 compounds of the dimer systems, some are dominated by their core-core interactions and others by their substituent-substituent interactions. By investigating potential energy hyperplanes and generating several different polymorphic conformers of the dimers, we gain detailed insight into the materials and the polymorphic character within the amorphous material.
In addition, trimer and tetramer agglomerates were calculated using the same procedure. We demonstrated that the average binding energy per molecule correlates well for the dimer, trimer, and tetramer systems. However, due to the different orientations of the substituents and the whole molecules, the geometric structures of the dimers is not directly comparable to the larger trimer and tetramer agglomerates.
Diskussion
The decomposition of the binding energy shows a strong correlation with the habit of the solid. Dimers with compounds dominated by their core-core interaction tend to form amorphous solids, while those with substituent-substituent dominated interactions prefer a crystalline or partially crystalline solid-state structure. Based on the analysis of trimer and tetramer systems, we conclude that the energetic results of the fundamental molecule-molecule interaction are transferable to larger systems and probably to solid-state materials. Our study represents the first step towards a better understanding of the structural properties and the energetics of cluster materials with an adamantane-like core structure, which exhibit unique non-linear optical properties.