Influence of Mixing Processes on the Performance of High-Pressure Polymerization I
Einleitung
In industrial low-density polyethylene (LDPE) production, mixtures of organic peroxide initiators are used to start the radical polymerization. The thermal decomposition rate of initiators varies with the functional groups of the peroxides and influences the radical concentration in the reactor, which again has an impact on the microstructure of the polymer. This connectivity is to be analyzed by calculating the radical distribution in a laboratory autoclave reactor by CFD simulations depending on the composition of the initiator mixture.
Methoden
The modelling approach combines two different methods - CFD and Monte Carlo - to link operating conditions like feed composition to polymer properties. The CFD model is based on a continuous stirred 100 mL reactor operating at 2000 bar and adiabatic conditions with a feed temperature of 70°C. Polyethylene is formed by free-radical polymerization of ethylene. A complex reaction mechanism is included which considers the main reactions initiation, propagation, and termination as well as transfer reactions to low and high molecular species, backbiting, β-scission, and propagation of terminal double bonds. Based on the CFD solution, the geometry is divided into multiple compartments, that are assumed to be ideal CSTRs connected by mass streams. With the average concentrations in each compartment, event frequencies for the reactions named above are calculated as an input for the Monte Carlo simulation.
Ergebnisse
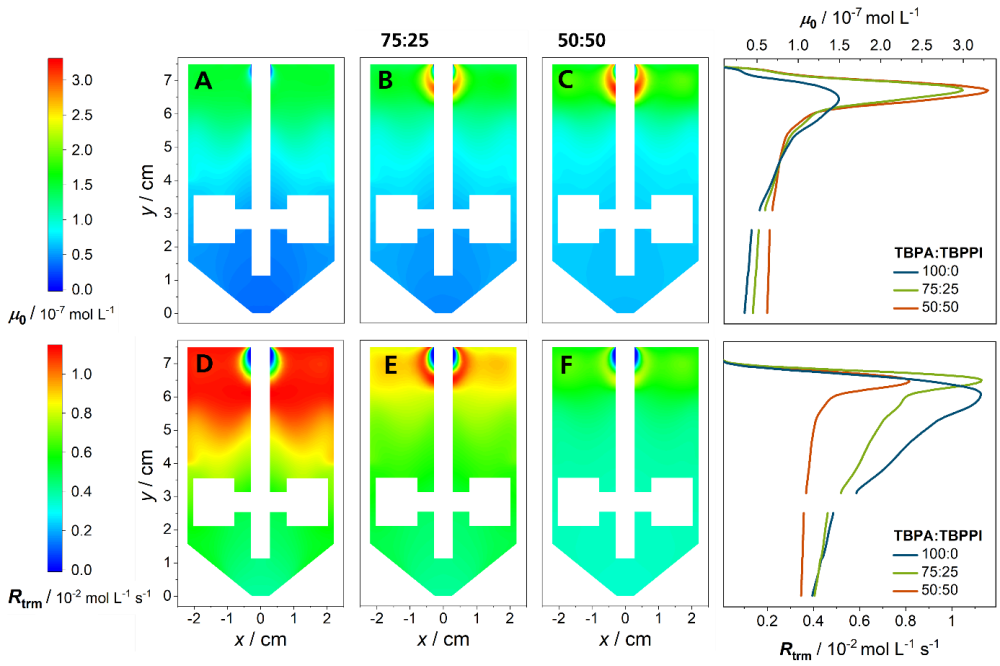
The composition of an initiator cocktail containing a high and a low temperature initiator is varied by keeping the total amount of initiator constant. In the figure above, the distribution of the zeroth moment of primary radicals is shown. With increasing amount of low temperature initiator, the radical concentration increases, especially close to the inlet. The termination reaction rate depends quadratically on the radical concentration, while the propagation reaction rate only depends linearly on the radical concentration. This leads to a lower monomer conversion. Due to the exothermic propagation reaction the temperature decreases as well. The transfer reaction rates are highly temperature dependent and consequently decrease. Regarding the polymer properties, lower short- and long-chain branching densities, as well as a shift of the molecular weight distribution to higher molecular weights are observed.
Diskussion
An open topic is the validation of the CFD results with experimental data. Due to the extreme process conditions (2000 bar, 200°C), laboratory plants are complex and expensive to operate and require high safety standards. Relevant information, such as concentration profiles of the reactive radical species, can hardly be determined experimentally. A possible approach is the comparison of the temperature at different positions in the reactor. Therefore, experiments under well-defined temperature conditions must be carried out. Furthermore, the experimental and simulated molecular weight distribution can be compared.