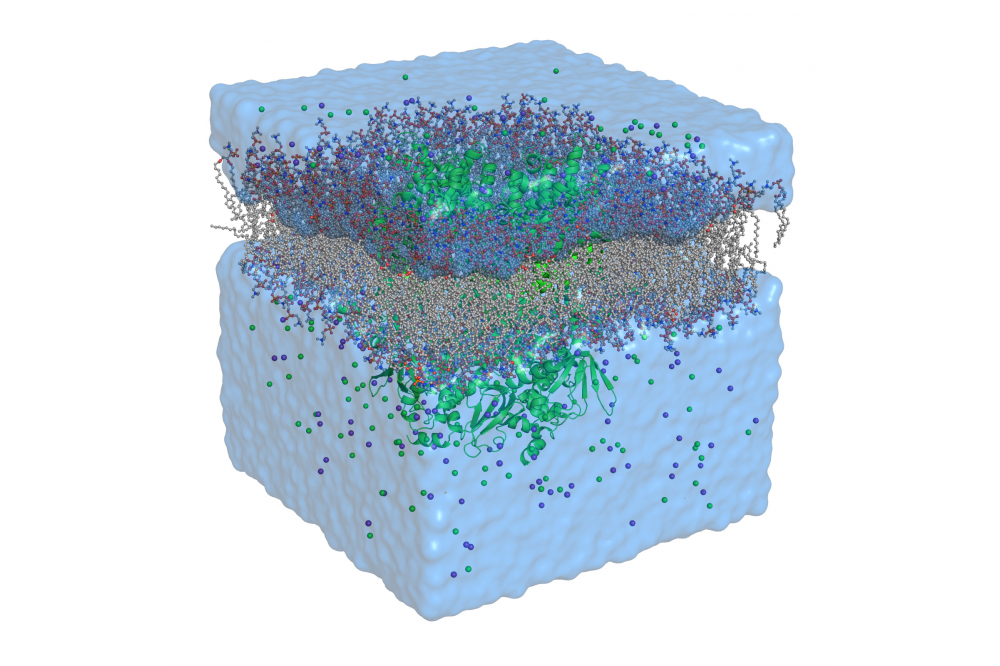
Molecular Dynamics Study of the Sodium/Potassium Channels HCN
Einleitung
Ion channels play a fundamental key role in all living organisms and are crucial for the signal transduction of neurons in higher animals. In human, the hyperpolarization-activated cyclic nucleotide-gated (HCN) sodium/potassium channels of the HCN family are crucial for various biological processes. In the neural and cardiovascular system, they are responsible for the pacemaker current (also known as funny current If ), which is characterized by a slow and rhythmic mixed sodium and potassium influx into cells. Mutations in the genes of the HCN family have been linked to various diseases, including rhythmias, epilepsies and neuropathic pain.
Even though discovered several decades ago, only little is known about why HCN channels act so different compared to their relatives (potassium selective channels). The recently solved 3D structures of multiple members of the HCN family allows us to use computational simulation techniques to investigate some of the key aspects of these channels: HCN channels are only weakly potassium selective and they activate at hyperpolarizing voltages.
Methoden
We use molecular dynamics (MD) simulations of protein/membrane systems to simulate potassium channels embedded into membrane systems. In this simulation method, the physical movement of atoms is solved using Newton’s equations of motion to approximate the dynamic evolution of the system. This is combined with enhanced sampling techniques to sample states that are usually not accessible by classical MD simulation as well as novel analysis tools.
Ergebnisse
During the first project period, we focused on the effects of naturally occurring mutations on the HCN1 channel structure and why specific mutations cause epilepsy. In combination with in-vitro experiments, we were able to show that a specific mutation inside the central pore of HCN1 can stop the channel from closing but also blocks ion conduction. In contrast, other mutations had a less severe effect on the channel. Therefore, we have been able to give a plausible explanation to the question why this specific mutation is linked to severe forms of epileptic encephalopathy in human. During the second period, we used the same techniques to investigate the structure-function relationship in HCN channels by introduction of rationally designed mutations. We were able to show that the HCN domain functionally links the cAMPand voltage-sensing domain to the central pore and thus plays a crucial role for channel gating. Also in this project period, we investigated how ions bind to the selectivity filter of HCN4 and revealed a novel conduction mechanism unique to this channel species.
Diskussion
Investigation of how HCN channels work in general is crucial for the development of drugs targeting HCN-related diseases like epilepsy. Additionally, insight into the structure-function relationship of larger ion channels like HCN represents a key step towards the development of tailored channels with defined characteristics. With our simulations, we can close the gap between static structural analysis and experimental biology. In the future, we plan to further our understanding of several key aspects of HCN channels including voltage-dependent gating and especially ion conduction and selectivity mechanisms.