Cononsolvency in Aqueous Alcohols Solutions
Einleitung
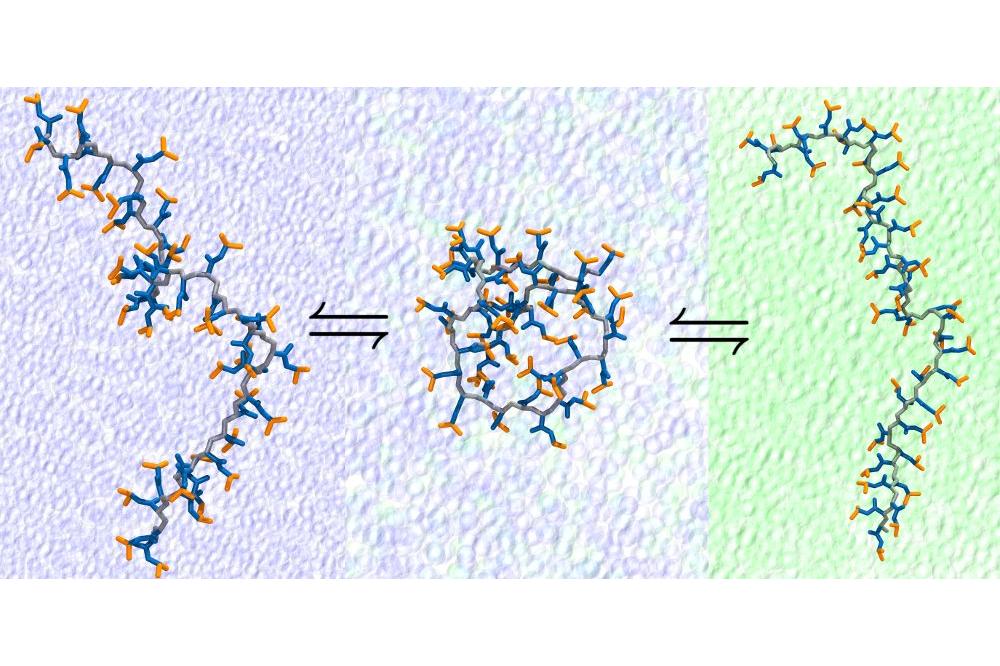
Smart polymers respond to environmental factors such as change in temperature, pH, or solvent. An example is the nonlinear dependence of solubility on the solvent composition where a mixture of two good solvents yield a poor one. This phenomenon is known as cononsolvency and it has been known for over 30 years. However, there is still no consensus over its molecular mechanism. A number of models have been suggested, some of which outright contradict with each other. The task to identify the responsible factors is impeded by a number of issues. For one, the experimental studies are mostly limited to larger scales where direct observation of the responsible molecular interactions is not easy. Although computational simulations allow identifying molecular interactions, they face the tremendous task of properly sampling highly flexible chains at large resolutions. Furthermore the subtle interplay between the enthalpic and entropic contributions and the shortcomings of the available methods to measure these components with high accuracy increases the difficulty of the task. The factors above prevent an easy setup to explain the underlying mechanism and therefore leads to a disagreement between the community.
Methoden
We used extensive molecular dynamics simulations coupled with enhanced sampling techniques to overcome the sampling barrier due to the highly flexible nature of the polymer chain. We developed recipes to decompose the energetic interactions and calculated the calorimetric data for the collapse transition. We tested various force fields for their accuracy in reproducing the experimental behavior.
Ergebnisse
Using extensive computational simulations to overcome the sampling problem of a highly flexible chain we found out that the collapse tendency of the polymer is highly correlated with enthalpic interactions. Decomposing the enthalpy yielded that the polymer-solvent interaction plays the determining role for the cononsolvency of PNIPAM in water/methanol mixtures compared to the polymer-cosolvent or the solvent-cosolvent interactions. Furthermore, the hydrogen bonding between the polymer and the solvent molecules is directly affected by the competition between the solvent and cosolvent for the available hydrogen bonding sites. Moreover, we showed that the current OPLS/AA forcefield which is widely used to study such systems fails to reproduce the temperature behavior for PNIPAM in aqueous solution. We modified the forcefield to yield a more representative model that matches the experimentally proven behavior of the polymer.