Investigation of the Cell Size Effect of CeO2 on the Calculated Raman Spectra of VOx/CeO2 Catalysts
Einleitung
Ceria (CeO2) is an important support material for transition metal based oxides like vanadia (VOx). These VOx/CeO2 catalysts are used in oxidation reactions like the partial oxidation of different alcohols and alkanes. [1] The ODH of propane is a reaction of great potential for industrial applications to synthesize propylene directly from propane as an alternative to steamcracking, for example. The process is not used on a large scale yet, since the reaction leads to low yields due to the combustion of propane to CO2. [2] This might be overcome by the catalytic oxidation using VOx/CeO2. To optimize the catalyst, the knowledge of the reaction mechanism for the ODH of propane over ceria supported vanadia catalysts is of great importance. To investigate the mechanism a combined approach of operando Raman spectroscopy and DFT calculations is employed. DFT calculations can be used to calculate Raman spectra of the VOx/CeO2 catalyst to aid the interpretation of the experimental operando Raman results. Since calculated Raman spectra for this system are completely new to literature, the influence of different cell sizes on the resulting spectrum was tested in this project.
Methoden
All calculations in this project were performed using the Vienna ab initio simulation package (VASP) (URL: http://cms.mpi.univie.ac.at/vasp). A license for the code is available for the group of Prof. Dr. Christian Hess. Structure optimizations were performed for ceria cells of p(2x2) and p(3x3) cell sizes with a VO3 cluster deposited on the surface and vibrational frequencies were calculated using density functional perturbation theory (DFPT). Raman intensities were calculated using a finite difference approach based on the dielectric tensor. A detailed description of the approach is given in reference [3].
Ergebnisse
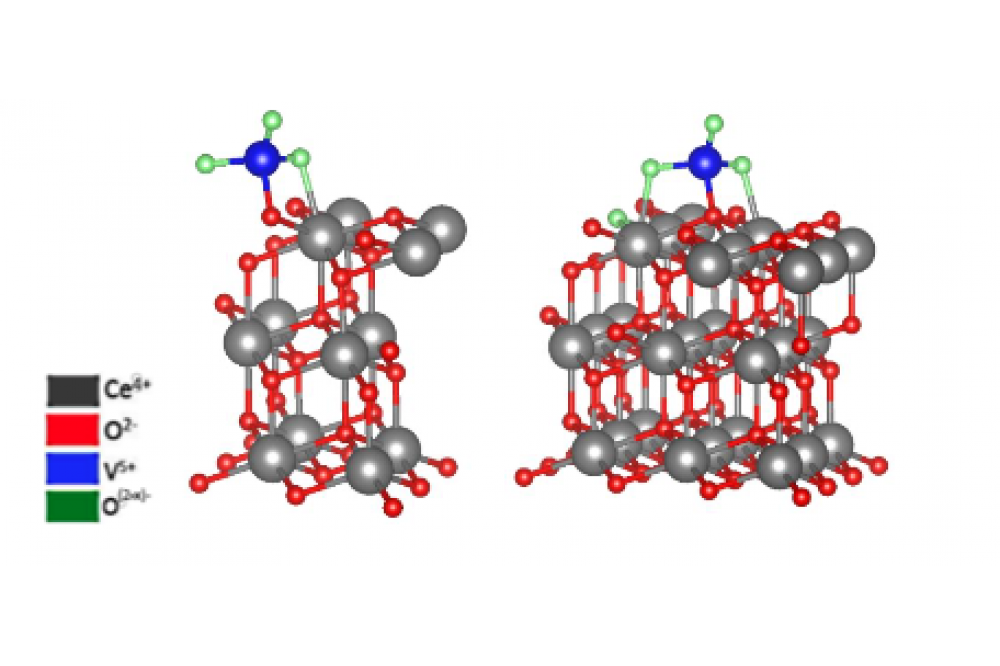
Structural relaxations were performed for ceria p(2x2) and p(3x3) cells with a deposited VO3 cluster as described above. The structures were relaxed until the residual forces were <0.01 eV/Å. The resulting structures are shown in figure 1.
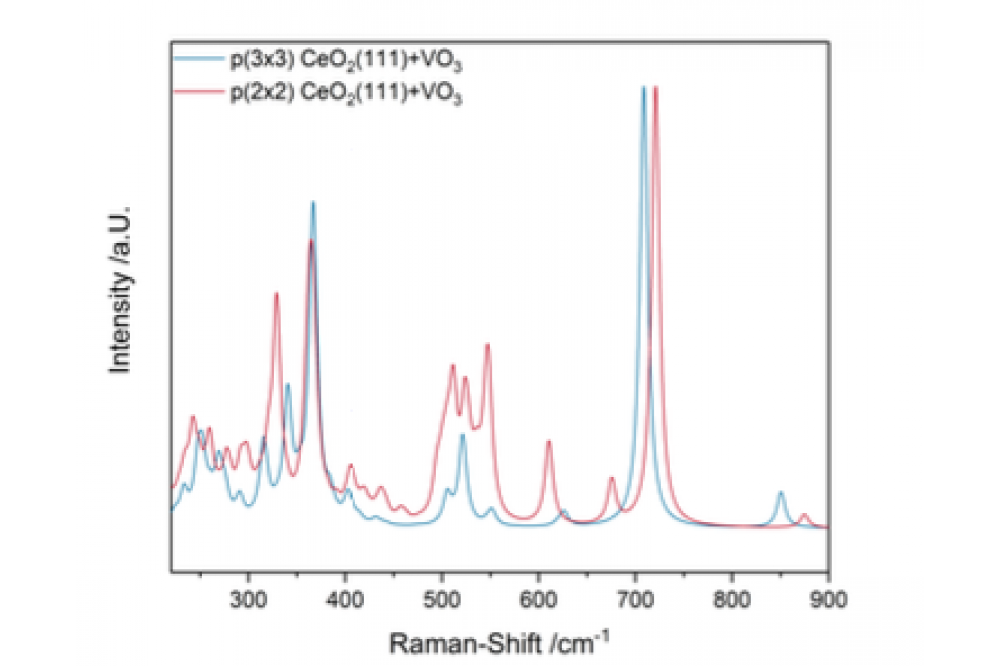
Normal mode analysis was performed for the structures shown in figure 1 and the Raman intensities were calculated based on the obtained frequencies as described in section 1.5.2. The resulting spectra are shown in figure 2.
Diskussion
Upon deposition of the VO3 cluster, the electronic structure of the ceria changes. Since the vanadium atom in the isolated VO3 cluster should have an oxidation state of +VI, but the ceria keeps the vanadium atom of the cluster deposited on the surface always at +V, one electron from oxygen 2p states is transferred to the vanadium atom. [4] It could be shown that the electronic structure change differs for p(2x2) and p(3x3) cells. The electron transferred to then vanadium atom has contributions from three oxygen atoms of the CeO2 for the p(2x2) cell and four oxygen atoms for the p(3x3) cell. These oxygen atoms are oxidized from –II to (–II+x) with x=0.33 for the p(2x2) and x= 0.25 for the p(3x3) cell. Based on another DFT project in our group (1024) similar structural differences could also be observed for VO and VO4 clusters. The total energy and the vibrational frequencies of the V-O vibration at ~850 cm-1 for the p(2x2) cell are in agreement with the literature [5] but the vibrational frequency for the V-O bond also differs between the cell sizes. The peaks are ~25 cm-1 apart. Furthermore, differences in the Raman spectra of the two different cells are observable. Apart from a shift for the same peaks (V-O at ~850 cm-1 and V-O-Ce at ~725 cm-1 ) of between 15 cm-1 and 25 cm-1, relative intensities also differ for the two cell sizes. Interphase bonds between 480 cm-1 and 650 cm-1 are more
intensive for the small cell size and an additional peak can be observed at 675 cm-1 . This peak is caused by an oscillation of the two V-O-Ce bonds of the two interphase oxygen atoms contributed by the VO3 cluster, which is only observable for the small cell size.
These findings may indicate, that the p(2x2) cell size is too small to accurately describe the vanadia monomers on the ceria surface. The monomeric species may interact with each other through the periodic boundary conditions. This might indicate that the vanadia loading on p(2x2) cells is too high and, in reality, the vanadia structures would be thermodynamically favoured to form dimeric or oligomeric species. Characteristic ceria vibrations like the F2g at 370 cm-1 and the Ce-O transversal surface phonon between 220 cm-1 and 300 cm-1 are also more pronounced for the p (3x3) cell. The broad band of the Ce-O surface phonon is caused by the participation of the VO3 cluster in multiple vibrations with different frequencies resulting in a broad region of Ce-O vibrations in comparison to literature results [6].These results lead to the conclusion, that the p(2x2) cells are approximations, which need less calculation time but the p(3x3) cells are more accurate and are therefore favoured for calculations of Raman spectra.